
ACT Science Practice Test 40
Thời gian làm bài: 1 giờ
Đề thi nằm trong bộ sưu tập: Tuyển Tập Bộ Đề Thi Đại Học Hoa Kỳ (ACT) - Có Đáp Án Chi Tiết
Hãy bắt đầu chinh phục nào!
Xem trước nội dung:
Radium-226 (226Ra) activity is often elevated in bodies of water that have been augmented with groundwater. Samples were collected over a two-year period in order to study 226Ra activity in Florida Marsh and Cypress wetlands. Figure 1 shows the average 226Ra activity from surface sediment samples collected in 2002 and 2004. Cores were taken from shallow sediment (0 to 4 cm) in the wetlands and analyzed for 226Ra activity. The activity, in disintegration per minute per gram (dpm/g), found in the Marsh and Cypress wetlands is displayed in Figures 2 and 3.
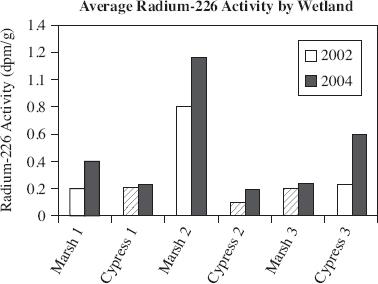
Figure 1
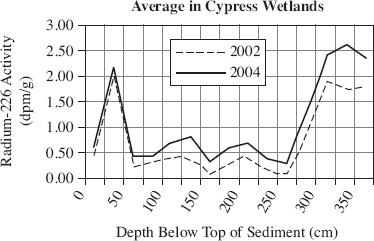
Figure 2
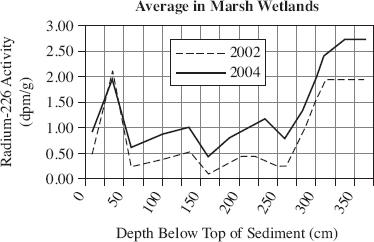
Figure 3
According to Figure 2, the average radium-226 activity in Cypress wetlands in 2004 at a depth of 200 cm was closest to which of the following?
0.30 dpm/g
0.70 dpm/g
1.00 dpm/g
2.50 dpm/g
Based on the information in Figure 3, if the average radium-226 activity had been measured in Marsh wetlands at the depth of 400 cm in 2002, it most likely would have been closest to which of the following?
2.75 dpm/g
2.50 dpm/g
2.00 dpm/g
1.80 dpm/g
Which of the following is the most likely explanation for the difference in the average 226Ra activity between 2002 and 2004?
In 2004 the wetlands were augmented with groundwater, which caused an increase in 226
In 2002 the wetlands were augmented with groundwater, and in 2004, they were not. This resulted in more 226
The amount of groundwater the wetlands were augmented with was higher in 2002 than in 2004.
In 2004 there was more rainfall than in 2002, which created a decrease in 226
If the data in Figures 2 and 3 are typical of Cypress and Marsh wetlands, one would most likely make which of the following conclusions about the 226Ra activity in a wetland?
Each year, the 226
According to Figures 2 and 3, the 226Ra activity increased between the depths of 200 and 250 cm for which wetlands?
Cypress wetlands in 2002
Cypress wetlands in 2004
Marsh wetlands in 2002
Marsh wetlands in 2004
The balanced chemical equation for the dissociation of acetic acid in water is shown below:
CH3COOH(aq)  CH3COO- (aq) + H+(aq)
CH3COO- (aq) + H+(aq)
The reaction can proceed in either direction in order to maintain a constant value for the acid dissociation constant. Acetic acid is an acid because it contributes H+ ions to the solution. The acid dissociation constant for the above reaction can be expressed as follows:
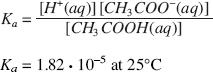
The expression [A] represents the concentration of substance A in moles/liter. One mole is 6.02 · 1023 molecules.
Experiment 1
NaOH is a strong base because it dissociates readily in water and contributes OH– ions to the water. A 1 M solution of NaOH, a strong base, is slowly added to a 100 ml, 2 M (M represents molarity, a measure of concentration in 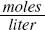 ) solution of acetic acid. The NaOH solution contributes OH– ions to the mixture. An ion is a charged atom or molecule. The OH– ions from the NaOH combine with the H+ ions from the acetic acid to form water as shown below:
) solution of acetic acid. The NaOH solution contributes OH– ions to the mixture. An ion is a charged atom or molecule. The OH– ions from the NaOH combine with the H+ ions from the acetic acid to form water as shown below:
H+(aq) + OH– (aq) → H2O (l)
Various measurements were taken and the results are shown in Table 1 and graphed in Figure 1. Note: pH = –log [H+]
Table 1
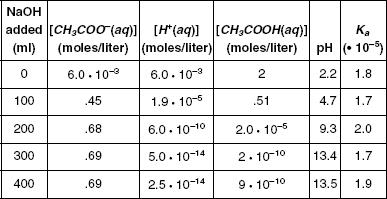
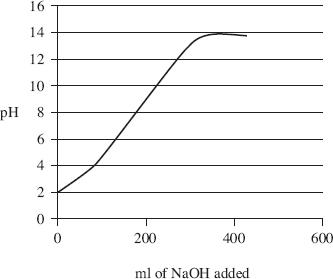
Figure 1
Using the figure and the table, how much NaOH was added in order to increase the pH to 9.3?
0 ml
100 ml
200 ml
300 ml
If only 50 ml of NaOH had been added, what approximate value would the pH have been?
1.5
3.5
5.5
7.5
Using all the information in the passage, why did the concentration of CH3COOH(aq) continue to decrease while the concentration of CH3COO–(aq) remained nearly unchanged?
Looking at the KaCH3COOHaqCH3COO–aqH+aqCH3COOH
Looking at the KaCH3COOHaqCH3COO–aqH+aqCH3COOH
The NaOH produces Na+CH3COOH
Clearly, the data must be inaccurate. Looking at the balanced equation, for every molecule of acetic acid that dissociates, one CH3COO–CH3COO–aq
What would be the effect on the results if 100 ml of 1 M acetic acid was used instead of 100 ml of 2 M?
The decreased concentration of acetic acid will decrease acidity and increase the initial p
The decreased concentration of acetic acid will decrease acidity and decrease the initial p
The increased concentration of acetic acid will increase acidity and increase the initial p
The increased concentration of acetic acid will increase acidity and decrease the initial p
How could you lower the pH back to 2.2?
Adding enough NaOH will eventually lower the pH because the cycle will repeat.
Adding enough water will dilute the base and lower the p
Evaporating some of the water will increase the concentration of H+
Adding a sufficient amount of acetic acid will add H+
What is true about the relationship between [H+(aq)] and pH?
There is an inverse relationship; as [H+aq
There is a direct relationship; as [H+aq
The relationship is complex. Because the concentrations are so small, the pH jumps after 200 ml of NaOH is added because small quantities of H+H+
There is no relationship between [H+aq
Air permeability is defined as the ability of soil to transmit air through interconnected air-filled pores under an imposed air pressure gradient. Air permeability is a function of volumetric water content, porosity, pore size distribution, and pore geometry. Scientists used a soil corer air permeameter (SCAP) to digitally measure the flow rates of air through desert soil under low-pressure gradients. The SCAP measured air permeability both in situ, with the instrument inserted in the soil and ex situ, after the soil corer had been removed from the soil. Figures 1 and 2 display the results of field testing conducted at two of the four sites in Arizona, over the course of three months. The porosity of the soil at each site is displayed in Figure 3. Figure 4 shows the relationship between the air pressure used and the flow rate of the air for each site.
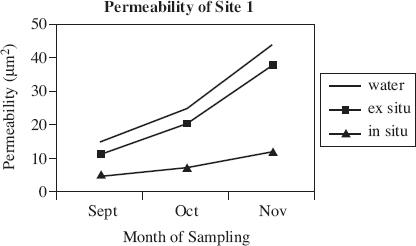
Figure 1
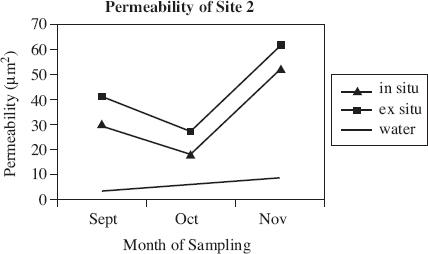
Figure 2
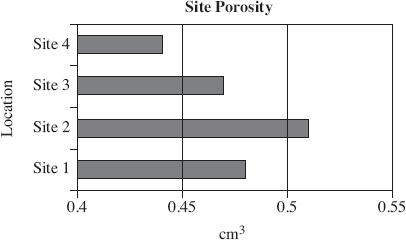
Figure 3
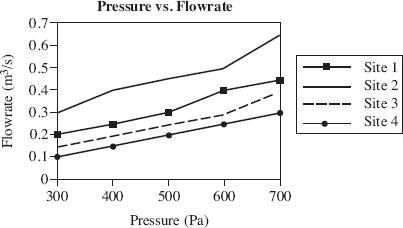
Figure 4
According to Figure 2, the air permeability measured ex situ in October was closest to which of the following?
5 μm2
15 μm2
20 μm2
30 μm2
Based on Figure 4, if the air permeability had been tested at Site 1 using 800 Pa, the flow rate would most likely have been closest to which of the following?
0.5 m3
0.6 m3
0.4 m3
0.3 m3
Which of the following is the most likely explanation for the difference in air permeability at Site 1 versus Site 2?
The overall air permeability is higher at Site 1 due to the lower porosity of the soil.
The overall air permeability is higher at Site 2 due to the higher porosity of the soil.
The overall air permeability at Site 2 is higher because less pressure was used when testing the soil.
The overall air permeability at Site 2 is higher because more pressure was used when testing the soil.
If the data in Figures 1 and 2 is typical of air permeability measurements, one would most likely make which of the following conclusions about air permeability in situ versus ex situ?
The permeability will always be higher ex situ than in situ.
The permeability will always be lower ex situ than in situ.
Water permeability is higher than air permeability in situ and ex situ.
At high pressures, air permeability is greater in situ than ex situ.
According to Figure 1, which of the following statements is most accurate regarding permeability during the month of November?
Permeability was lower than in previous months.
Permeability was highest for air measured while the SCAP was still inserted in the ground.
Permeability was highest for air measured after the SCAP had removed a core from the soil.
Permeability was highest for water.
Scientists studying sucrose examined oranges and lemons to determine how the two fruits form and synthesize sucrose. Studies were conducted both on extractions from the fruits and on small, intact fruits.
Study 1
Mature lemon and orange fruits were obtained and then juiced by hand. Formation of fructose was determined using two portions of the fruits: the juice, and the particulate sediment. The results are shown in Table 1.
Table 1—Formation of Sucrose by Fructose Reaction
| Preparation | Sucrose Synthesized (μ moles) |
| Oranges Juice Particulate sediment | 0.12 0.9 |
| Lemons Juice Particulate sediment | 0.4 0.17 |
Study 2
Scientists found lemons of varying ages from the same fruit grove. Each piece of fruit was cut into sections, and various tissue samples were isolated for testing. The three tissue types tested were the flavedo (colored, outer layer of the peel), the albedo (white peel layer), and the vesicle (fleshy part of the fruit). The results of the formation of sucrose in lemons are shown in Figure 1.
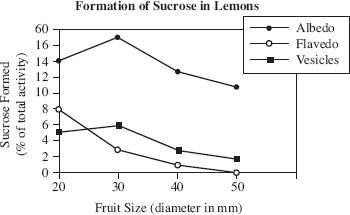
Figure 1
Study 3
Whole fruits (each weighing approximately 5 g) were tested and then cut into sections to measure sucrose activity in the various tissues. Figure 2 displays the results.
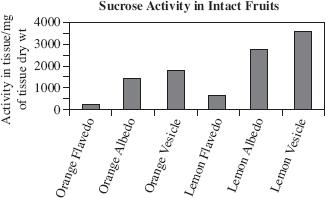
Figure 2
Based on Figure 1, which type of lemon tissue forms the most sucrose?
The albedo tissue in a large lemon
The albedo tissue in a small lemon
The flavedo tissue in a large lemon
The vesicles in a small lemon
According to the results of Study 2, as fruit size increased, the sucrose found in the vesicles:
increased only.
increased, then decreased.
decreased only.
decreased, then increased.
Based on the results of Study 3, the largest difference in sucrose activity was found in:
oranges between the flavedo tissue and the albedo tissue.
oranges between the albedo tissue and the vesicle tissue.
lemons between the flavedo tissue and the vesicle tissue.
lemons between the flavedo tissue and the albedo tissue.
Based on the results of Study 1, which of the following is most accurate about the formation of sucrose?
Orange juice is more effective at forming sucrose than lemon juice.
Orange juice is less effective at forming sucrose than lemon juice.
Lemon juice and orange juice are equally effective at forming sucrose.
Lemon juice forms less sucrose than lemon particulate sediment.
Assume Study 2 was repeated using oranges instead of lemons. Based on the information presented in Figures 1 and 2, and assuming that the fruits used in Study 3 were 30 mm in diameter, which of the following would most likely be the sucrose formation (as a percent of total activity) in the albedo tissue of the oranges?
0
3
9
20
Based on the results of Study 2, which statement most accurately summarizes the formation of sucrose in lemons?
The fleshy part of the lemon formed the majority of the sucrose.
The colored outer layer of the peel does not form any sucrose.
The part of the lemon that forms the most sucrose is dependent on the size of the lemon.
More sucrose is formed in the white peel layer of a lemon than anywhere else.
Xem thêm đề thi tương tự

12 câu hỏi 1 mã đề 1 giờ
214,143 lượt xem 115,283 lượt làm bài

24 câu hỏi 1 mã đề 1 giờ
219,455 lượt xem 118,132 lượt làm bài

26 câu hỏi 1 mã đề 1 giờ
219,214 lượt xem 118,020 lượt làm bài

22 câu hỏi 1 mã đề 1 giờ
219,728 lượt xem 118,293 lượt làm bài

12 câu hỏi 1 mã đề 1 giờ
218,864 lượt xem 117,831 lượt làm bài

24 câu hỏi 1 mã đề 1 giờ
219,350 lượt xem 118,090 lượt làm bài

24 câu hỏi 1 mã đề 1 giờ
218,456 lượt xem 117,614 lượt làm bài

22 câu hỏi 1 mã đề 1 giờ
208,288 lượt xem 112,133 lượt làm bài

29 câu hỏi 1 mã đề 1 giờ
215,754 lượt xem 116,158 lượt làm bài
