
ACT Science Practice Test 46
Đề thi nằm trong bộ sưu tập: Tuyển Tập Bộ Đề Thi Đại Học Hoa Kỳ (ACT) - Có Đáp Án Chi Tiết
Số câu hỏi: 24 câuSố mã đề: 1 đềThời gian: 1 giờ
219,391 lượt xem 16,870 lượt làm bài
Xem trước nội dung:
Carboxylic acids are organic compounds containing a carboxyl (-COOH) group. These molecules are acidic since they are able to donate protons in solution. The acidity and other physical properties of carboxylic acids are affected by the composition of the atoms bound to the carboxyl group. Table 1 lists the freezing points and boiling points for several carboxylic acids.
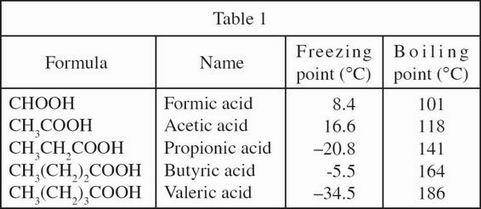
Figure 1 shows how the vapor pressure (in mm Hg) of 3 carboxylic acids changes as a function of temperature.
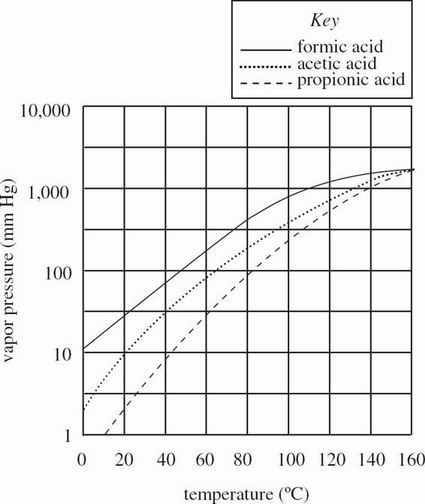
Figure 1
Figure 2 shows how the vapor pressure of the same 3 carboxylic acids changes as a function of concentration when mixed with water at 20°C.
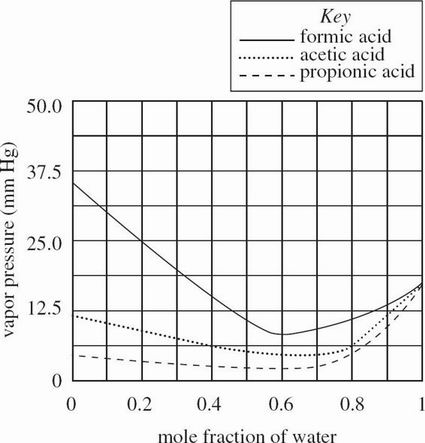
Figure 2
Which of the carboxylic acids listed in Table 1 has the highest melting point?
Propionic acid
Valeric acid
Acetic acid
Formic acid
According to Figure 2, the vapor pressure of a 0.5 mole fraction solution of water in formic acid is closest to the vapor pressure of which of the following water in formic acid solutions?
0.9 mole fraction
0.8 mole fraction
0.6 mole fraction
0.4 mole fraction
According to Figure 2, as the mole fraction of water in an acetic acid and water solution increases from 0 to 1, the vapor pressure:
decreases, then increases.
increases, then decreases.
decreases only.
increases only.
CH3 (CH2)4 COOH is the chemical formula for the carboxylic acid named hexanoic acid. Based on Table 1, this compound most likely boils at a temperature:
lower than 160°
between 200°C and 220°
between 220°C and 240°
higher than 240°
According to Figure 1, does acetic acid or formic acid resist vaporization more at 60°C ?
Formic acid, because formic acid has the lower vapor pressure.
Formic acid, because formic acid has the higher vapor pressure.
Acetic acid, because acetic acid has the lower vapor pressure.
Acetic acid, because acetic acid has the higher vapor pressure.
A solenoid is a device that creates a magnetic field from electric current and can be used to exert a force on a nearby bar magnet to activate a mechanical device.
Scientists performed experiments on the solenoid apparatus shown in Figure 1.
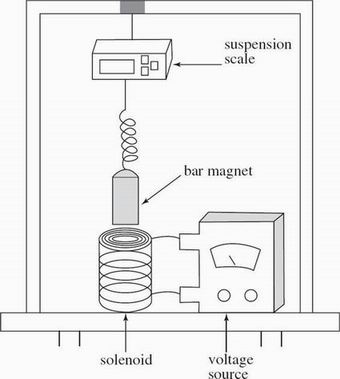
Figure 1
A wire carrying current from a voltage source was coiled into a hollow cylinder to form a solenoid with a length of XY. A solid cylinder bar magnet was suspended near the top of the solenoid as shown in Figure 2.
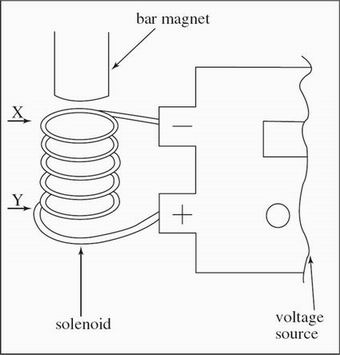
Figure 2
When the voltage source was turned on, the solenoid exerted a measurable force on the suspended bar magnet.
The bar magnet was attached to a digital suspension scale that measured weight in newtons (N). With the voltage source off, the scale read 4.7 N. Prior to the start of each experimental trial, the scale was adjusted to read 5.0000 N.
Experiment 1
The scientists applied various levels of voltage in volts (V) to the circuit and recorded the weight indicated by the suspension scale for each trial. Results were recorded in Table 1.
Table 1
Voltage (V) Weight (N)
7.25 5.0078
8.00 5.0095
8.75 5.0113
Experiment 2
The scientists removed the bar magnet, inverted it, and reattached it to the suspension scale so that the opposite end was now facing the solenoid. The procedures of Experiment 1 were repeated and results were recorded in Table 2.
Table 2
Voltage (V) Weight (N)
7.25 4.9922
8.00 4.9905
8.75 4.9887
Experiment 3
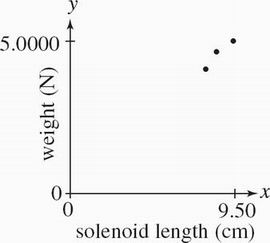
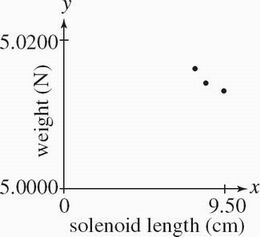
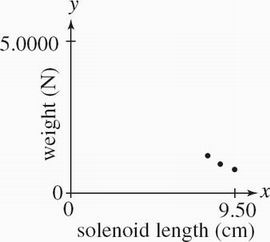
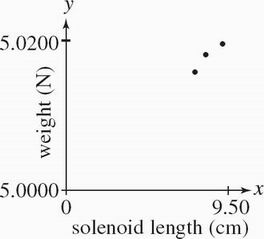
The bar magnet was returned to the original alignment it was in during Experiment 1. The length XY of the solenoid coil was varied while a voltage of 8.00 V was applied to the circuit. Weights were recorded in Table 3.
Table 3
Solenoid length XY (cm) Weight (N)
9.50 5.0105
8.50 5.0131
7.50 5.0169
Based on the results of Experiments 1 and 3, the length XY of the solenoid coil in Experiment 1 was most likely:
shorter than 7.50 cm.
between 7.50 cm and 8.50 cm.
between 8.50 cm and 9.50 cm.
longer than 9.50 cm.
In Experiments 1 and 2, the orientation of the bar magnet relative to the solenoid opening determined which of the following?
Solenoid length XY
Direction of the force exerted by the solenoid on the bar magnet
Density of the bar magnet
Magnetic field strength of the solenoid
Which of the following provides the best explanation for the results of Experiment 3 ? The force exerted on the bar magnet by the solenoid magnetic field:
decreased as the voltage applied to the circuit decreased.
increased as the voltage applied to the circuit decreased.
decreased as the length XY of the solenoid decreased.
increased as the length XY of the solenoid decreased.
Suppose the scientists maintained the same bar magnet orientation in Experiment 3 as in Experiment 2. Based on the results of Experiments 1 and 2, with the solenoid length XY equal to 9.50 cm, the weight on the scale would most likely have been:
5.0169
5.0105
4.9895
4.9831
Prior to all experiments, the suspension scale was calibrated to read exactly 0 N when nothing was attached. Once the bar magnet was attached, the scientists made which of the following adjustments to the scale reading for each of the experimental trials?
The displayed weight was adjusted downward by approximately 1.3
The displayed weight was adjusted upward by approximately 1.3
The displayed weight was adjusted downward by approximately 0.3
The displayed weight was adjusted upward by approximately 0.3
Which of the following graphs best depicts the results of Experiment 3 ?
Average global temperature is influenced by multiple different factors and has gone through significant changes throughout the history of the earth.
Global temperature affects Earth's oceans. In general, as the average global temperature increases, glacial and continental ice coverage decreases from melting. This combined with the thermal expansion of the oceans results in rising sea levels. The sea level present at any given time over the past 150,000 years can be estimated from sedimentary rock layer analysis. Figure 1 shows for the years listed the difference in average global sea level compared to what has been the average temperature value from 1961 through 1990.
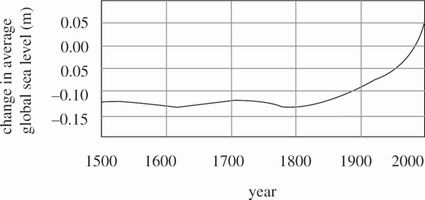
Figure 2
Figure 1 adapted from "Intergovernmental Panel on Climate Change Fourth Assessment Report 2007."
Figure 2 shows the change in both the average global temperature and average global sea level from the present. The change in average global sea level is determined by:
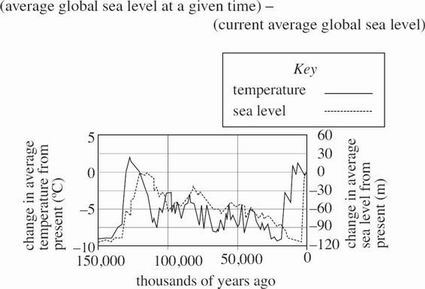
Figure 2
Figure 2 adapted from Petit et al. "Climate and atmospheric history of the past 420,000 years from the Vostok ice core," Antarctica. Nature 399: 429–436.
Two scientists discuss why the average global sea level has risen over the past 100 years.
Scientist 1
Rising sea levels are a direct result of widespread industrial burning of fossil fuels that started in the mid-19th century. This activity caused the release of greenhouse gases like carbon dioxide, which raised the average global temperature. This increase in temperature led to depleted ice coverage and rapid thermal expansion of oceans. Figure 1 shows that global sea level was essentially constant until approximately 1900 and has since risen to a level higher than at any time in the past 150,000 years. Between 1950 and 2000, global average sea level rose by 0.1 m as global temperature rose approximately 0.5°C. Since 2000 the change in global average sea level has increased at a rate of 2% per year.
Scientist 2
Rises and falls in the global average sea level occurred many times in the past 150,000 years and well before the industrial revolution of the mid-1800s. In general, global average sea level rises and falls with global average temperature, as shown in Figure 2. Industrial activity did not begin until the 1800s and could not have been responsible for the changes in average global temperature and sea level before that time. The recent rise in sea level in response to global temperature is not significantly different from those before 1800, so human activity does not by itself have a measurable effect on average global sea level.
According to Figure 2, over which of the following time intervals did the average global sea level increase more than 100 times as much as Scientist 1 claims it did between 1950 and 2000 ?
Between 130,000 and 120,000 years ago
Between 80,000 and 70,000 years ago
Between 50,000 and 30,000 years ago
Between 40,000 and 20,000 years ago
Which of the following statements about average global temperature would most likely be supported by Scientist 2 ?
Average global temperature has remained essentially constant for the past 150,000 years.
Average global temperature is changed only by widespread industrial activity.
As the average global temperature decreases, average global sea level decreases.
As the average global temperature decreases, average global sea level increases.
According to Scientist 1, the change in average global sea level has been increasing at a constant rate since 2000. Given the change in sea level given in Figure 1 for the year 2000, Scientist 1 would most likely conclude that the average global sea level change for the year 2003 was closest to which of the following?
.046 m
.048 m
.051 m
.053 m
Scientist 1 states that average global sea level is currently higher than at any point in the past 150,000 years. Does Figure 1 provide sufficient basis for this statement?
No; Figure 1 shows the change in average global sea level for the past 500 years only.
No; Figure 1 shows the change in average global sea level for the past 150,000 years.
Yes; Figure 1 shows the change in average global sea level for the past 500 years only.
Yes; Figure 1 shows the change in average global sea level for the past 150,000 years.
Assume that the current average sea level at a particular location is measured at 90 m above a fixed geographic landmark. Given Figure 2, the two scientists would most likely claim that 100,000 years ago, the average sea level above the same mark at that location was closest to which of the following?
120 m
100 m
60 m
30 m
Suppose Scientist 2 stated that there have been times over the past 150,000 years when average global sea level has been the same as what it is today. To support this claim, Scientist 2 would most likely cite the sea level data in Figure 2 for which of the following times?
50,000 years ago
65,000 years ago
115,000 years ago
130,000 years ago
Given Figure 1, Scientist 1 would most likely claim that from 1500 to 1800, average global temperature:
varied by less than 1°C, remaining essentially constant.
varied by 1°
increased by more than 1°
decreased by more than 1°
The force per unit area resulting from the separation of solutions of different concentrations by a selectively permeable membrane is called osmotic pressure. Molecules, including water, have a tendency to move from regions of high concentration to regions of low concentration. Selectively permeable membranes act as filters, only allowing molecules below a certain threshold size to pass through. Osmotic pressure is the pressure required to stop water from moving across such a membrane from a region of high to low water concentration.
Cupric ions (Cu2+) and glucose were dissolved separately in equal volumes of water to make two solutions. The glucose solution was more dilute, meaning that it had a higher percentage of water molecules than the cupric ion solution. Of the three molecules used for the solutions, water is the smallest and glucose is the largest. Water and glucose solutions are colorless while cupric ion solutions are blue. However, mixing glucose and cupric ions results in a red solution.
Experiment 1
A U-shaped tube contains a selectively permeable membrane, dividing it into equal halves. Glucose solution is poured in the left and an equal volume of cupric ion solution is poured in the right. Over 2 hours, the water level fell on the left and rose on the right. At this time, the left-sided solution was red and the right-sided was blue.
Experiment 2
Cupric ion solution is poured in the left and an equal volume of pure water is poured in the right. Over 2 hours, the water level fell on the right and rose on the left. At this time, both sides of the tube contained blue-colored solutions.
Experiment 3
Glucose solution is poured in the left and an equal volume of pure water is poured in the right. Over 2 hours, the water level fell on the right and rose on the left. At this time, both sides of the tube contained colorless solutions.
Albumin molecules do not pass through the selectively permeable membrane used in Experiments 1–3 and form clear solutions in water. If Experiment 2 were repeated, but the left side was filled with an albumin solution, the solution levels would:
fall on the left and rise on the right, resulting in a left-sided red solution and right-sided clear solution.
fall on the right and rise on the left, resulting in red solutions on both sides.
fall on the left and rise on the right, resulting in red solutions on both sides.
fall on the right and rise on the left, resulting in clear solutions on both sides.
In Experiments 1 and 2, cupric ion particles were able to move:
through the membrane into both the glucose solution and pure water.
through neither membrane into neither the glucose solution nor the pure water.
only through the membrane separating it from the glucose solution.
only through the membrane separating it from pure water.
In Experiments 2 and 3, what did the left side of the U-tube contain at the start of the experiment?
Experiment 2 Experiment 3
Cupric ion solution Pure water
Glucose solution Pure water
Cupric ion solution Glucose solution
Glucose solution Cupric ion solution
In Experiment 1, if the selectively permeable membrane allowed cupric ions, glucose, and water molecules all to pass, how would the results have differed, if at all?
The water level would have fallen on the right and risen on the left.
A red color would have appeared on both sides of the U-tube.
A blue color would have appeared on both sides of the U-tube.
The same results would have been observed.
After watching Experiment 1 only, an observer asserted that since the left-sided solution ended up red, cupric ions must be bigger than water molecules. Is this a valid assertion?
No; the results show only that cupric ions and water molecules are smaller than glucose molecules.
No; the results show only that cupric ions and water molecules are larger than glucose molecules.
Yes; the results show that water molecules but not cupric ions can pass through the selectively permeable membrane.
Yes; the results show that both water molecules and cupric ions can pass through the selectively permeable membrane.
In Experiment 1, before the molecules began to move relative to the semi-permeable membrane, the appearance of the right-sided solution in the U-tube was:
clear.
blue.
red.
purple.
