
ACT Science Practice Test 44
Thời gian làm bài: 1 giờ
Đề thi nằm trong bộ sưu tập: Tuyển Tập Bộ Đề Thi Đại Học Hoa Kỳ (ACT) - Có Đáp Án Chi Tiết
Hãy bắt đầu chinh phục nào!
Xem trước nội dung:
Groundwater is water stored beneath the surface of the Earth. Groundwater chemistry in 2 bodies of water—drawn from an aquifer and from beneath a wetland—was studied during a 2000 summer drought and again during the next summer, which had normal rainfall. Figure 1 shows the methane (CH4) gas concentration in the groundwater at various depths in the aquifer and wetland. Figures 2 and 3 show the groundwater conductivity (directly proportional to the concentration of the dissolved ions) and pH at various depths in the aquifer and wetland, respectively. Also shown are the locations of the water table and the depths of the aquifer and wetland below this level.
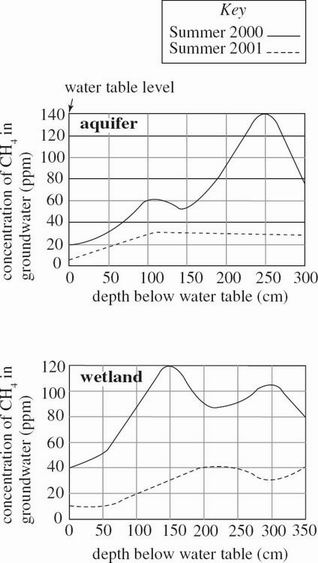
Figure 1
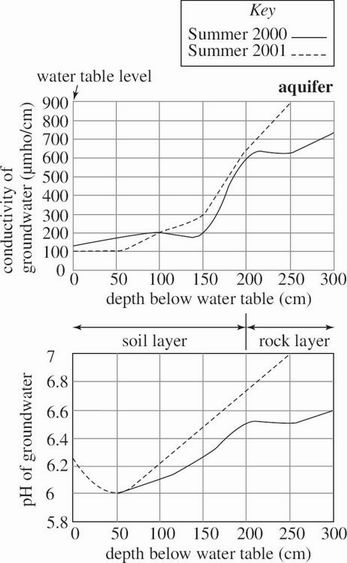
Figure 2
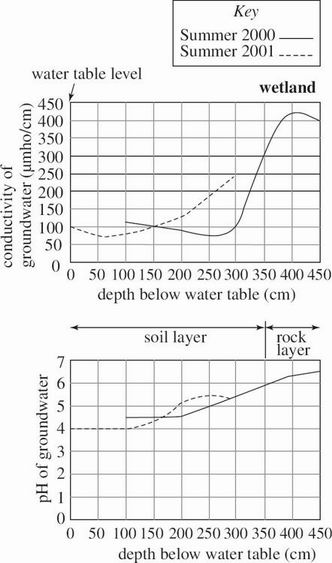
Figure 3
According to Figure 2, the conductivity of aquifer groundwater in 2000 at a depth of 250 cm was closest to which of the following?
350 μmho/cm
475 μmho/cm
625 μmho/cm
725 μmho/cm
Based on Figure 2, if the pH of aquifer groundwater at a depth of 260 cm had been measured in the summer of 2001, it would most likely have been closest to which of the following?
4.2
5.5
7.2
8.5
Which of the following is the most likely explanation for the difference in the depth of wetland groundwater in the 2 years?
The amount of groundwater discharged to the wetland was higher during the drought, so the wetland received more water than normal.
The amount of groundwater discharged to the wetland was higher during the drought, so the wetland received less water than normal.
The amount of rainfall received by the wetland was higher during the drought, so the wetland received more water than normal.
The amount of rainfall received by the wetland was lower during the drought, so the wetland received less water than normal.
If the data in Figures 2 and 3 are typical of aquifers and wetlands in general, one would most likely make which of the following conclusions about the soil layer in an aquifer and in a wetland?
The soil layer in both an aquifer and a wetland is completely above the water table at all times.
The soil layer in both an aquifer and a wetland is completely below the water table at all times.
The soil layer in an aquifer is thicker than the soil layer in a wetland.
The soil layer in an aquifer is thinner than the soil layer in a wetland.
According to Figure 1, the average concentration of CH4 over the depths of 0 to 300 cm was higher during the summer of:
normal rainfall than during the summer of drought in both the aquifer and the wetland.
normal rainfall than during the summer of drought in the aquifer only.
drought than during the summer of normal rainfall in both the aquifer and the wetland.
drought than during the summer of normal rainfall in the wetland only.
Polyatomic ions can be represented by the combination of symbols
(XaZb)n
where a is the number of atoms of Element X, b is the number of atoms of Element Z, and n is the total positive or negative charge for the entire polyatomic ion. If a or b is equal to 1, then the number 1 is omitted. If n is equal to 0, indicating a net neutral charge, then the number 0 is also omitted. For example, (NH4)+1 represents an ammonium ion, which contains 1 nitrogen atom (the number 1 is omitted), 4 hydrogen atoms, and a net polyatomic ion charge of +1. Since polyatomic ions carry a charge, they are very soluble in water, as opposed to neutral molecules.
Some atoms that comprise polyatomic ions are able to donate or accept different numbers of electrons, depending on the atoms with which they interact. In these different situations, they are said to have different oxidation states. For instance, while oxygen (O) typically has a constant oxidation state of -2, meaning it typically only accepts 2 extra electrons, chlorine (Cl) can have oxidation states of -1, +1, +3, +5, and +7, meaning that it has the ability to either accept 1 extra electron (-1) or donate either 1, 3, 5, or 7 electrons. The specific ions that feature the different oxidation states of chlorine are listed below.
| Table 1 | ||
| Name | Ion | Oxidation state of chlorine atom |
| Chloride | (Cl)−1 | −1 |
| Hypochlorite | (ClO)−1 | +1 |
| Chlorite | (ClO2)−1 | +3 |
| Chlorate | (ClO3)−1 | +5 |
| Perchlorate | (ClO4)−1 | +7 |
The energy required to remove an electron from an atom, thereby giving that atom a more positive oxidation state, is known as an ionization energy. The ionization energies for removing each of the first four electrons from elements with atomic numbers 11-15, as measured in electron-volts (eV), are shown in Figure 1.
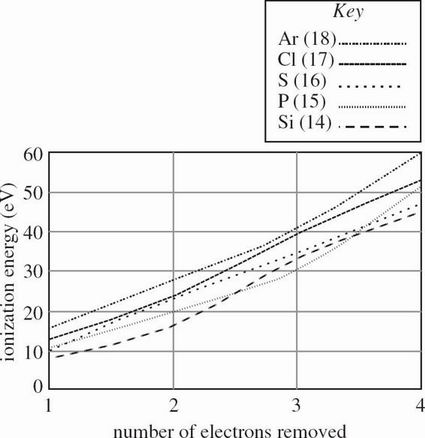
Figure 1
Which of the following symbols correctly represents the negatively charged polyatomic ion containing seven oxygen atoms and two chromium (Cr) atoms?
(CrO4-1
(Cr22-7
(Cr72-2
(Cr27-2
According to Table 1, what is the total charge for the polyatomic ion chlorite?
-1
+1
+2
+3
Based on Figure 1, the ionization energy required to remove 4 electrons from P (atomic number 15) is approximately twice the ionization energy required for which of the following?
Removing 1 electron from S
Removing 2 electrons from Ar
Removing 3 electrons from Si
Removing 4 electrons from Cl
A sample of bleach contains a mixture of chlorite and hypochlorite. Based on Table 1 and Figure 1, what is the ionization energy for the chlorine atom in each of these polyatomic ions?
Chlorite: IE of chlorine = 24 eV, Hypochlorite: IE of chlorine = 53 eV
Chlorite: IE of chlorine = 53 eV, Hypochlorite: IE of chlorine = 24 eV
Chlorite: IE of chlorine = 13 eV, Hypochlorite: IE of chlorine = 40 eV
Chlorite: IE of chlorine = 40 eV, Hypochlorite: IE of chlorine = 13 eV
Suppose a chloride ion is isolated and accelerated at a constant rate. How would the net force acting on the chloride ion compare with the net force acting on a perchlorate ion that is accelerated at the same constant rate?
It would be smaller, because chloride is more massive than perchlorate.
It would be smaller, because chloride is less massive than perchlorate.
It would be larger, because chloride is more massive than perchlorate.
It would be larger, because chloride is less massive than perchlorate.
Simple diffusion (SD) is the process by which an uncharged solute in water migrates directly across an uncharged membrane, while facilitated diffusion (FD) is the process by which a charged or polar solute travels through a channel or transporter that crosses the membrane. Figure 1 illustrates how two solutes can diffuse, one by SD and one by FD.
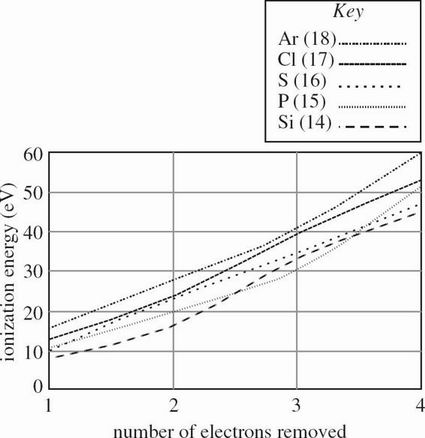
Figure 1
Solutes that cross a membrane by SD or by FD show different rates of flow across a membrane, also known as flux. As a solute crosses a membrane by SD, the flux follows a linear pattern over time, with smaller solutes having the greatest increase in flux over time. As a solute crosses a membrane by FD, the flux follows a logarithmic pattern, leveling off at a maximum flux since there are only a limited number of channels or transporters through which the solute can travel.
Experiment 1
One scientist introduced five different solutes of the same concentration to similar membranes at a constant temperature. The molecular masses of these solutes are shown in Table 1.
| Table 1 | |
| Solute | Molecular mass (amu) |
| #1 | 160 |
| #2 | 800 |
| #3 | 2,000 |
| #4 | 10,000 |
| #5 | 40,000 |
This scientist then measured the time it took for the solute to reach equilibrium, which is a state of equal concentration of the solute on both sides of the membrane. The results are shown in Figure 2.
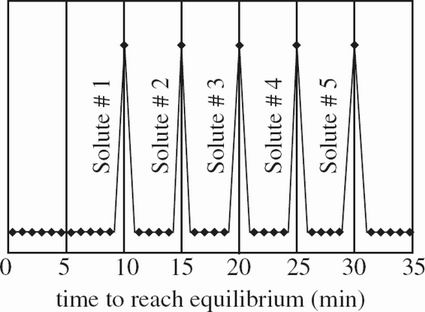
Figure 2
Experiment 2
Mixtures of solutes are subsequently introduced near three different membranes with different properties. The results of these three trials are presented in Figure 3.
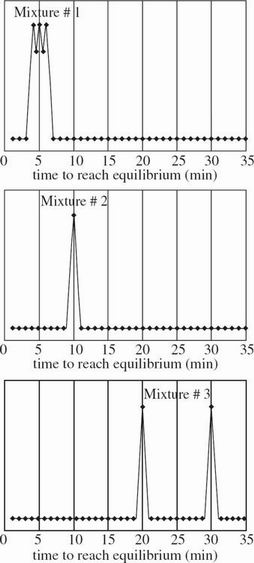
Figure 3
Based on the results of Experiments 1 and 2, Mixture #3 is likely to consist of which solutes from Experiment 1 ?
Solute #1 only
Solutes #1 and #3 only
Solutes #3 and #5 only
Solutes #2, #4, and #5 only
In Experiment 1, which solute spends the least amount of time flowing across the membrane before reaching equilibrium?
Solute #1
Solute #2
Solute #3
Solute #4
Based on the results of Experiments 1 and 2, which of the following ranks Solute #3, Solute #4, and Mixture #2 in order of smallest to largest average molecular mass?
Solute #3, Solute #4, Mixture #2
Solute #4, Mixture #2, Solute #3
Mixture #2, Solute #3, Solute #4
Mixture #2, Solute #4, Solute #3
In Experiment 1, on average, did molecules of Solute #3 or molecules of Solute #4 more easily diffuse across the membrane?
Solute #3, because it has a larger molecular mass.
Solute #3, because it has a smaller molecular mass
Solute #4, because it has a larger molecular mass.
Solute #4, because it has a smaller molecular mass.
In which mixture is the molecular mass most likely less than 160 amu ?
Mixture 1
Mixture 2
Mixture 3
Neither Mixture 1, 2, or 3
How does the number of molecules in 1 gram of Solute #1 compare with the number of molecules in 1 gram of Solute #5 ? The number of molecules in 1 gram of Solute #1 is:
less, because Solute #1 has a larger molecular mass than Solute #5.
less, because Solute #1 has a smaller molecular mass than Solute #5.
more, because Solute #1 has a larger molecular mass than Solute #5.
more, because Solute #1 has a smaller molecular mass than Solute #5.
The term "evolution" is often used in the context of biological changes in organism populations over time, but it can also be applied to the change in the chemical composition of the Earth's atmosphere. The hypotheses of two studies claim that this chemical evolution has altered the types of chemicals found in the atmosphere between the early stages of Earth's existence and the present day.
Study 1
Based on the hypothesis that volcanic eruptions were the source of gases in the early Earth's atmosphere, scientists recreated four model volcanic eruptions in closed chambers, each containing different percentages of the same volcanic particulate matter. They then observed the gases in the air above this model over time. The percent composition of this air after 1 day, when the air achieved a steady state of constant gas concentrations, is represented in Table 1.
Since the experiment provided only a suggestion of the gas levels in the early Earth's atmosphere, the scientists then analyzed the amount of trapped gases in sediment layers, which indicate the changing atmospheric levels of gases over billions of years. The data collected on O2 and H2O vapor are presented in Figure 1.
Study 2
A separate study used the same volcanic models as in Study 1, but it hypothesized that the scientists in Study 1 underestimated the amount of H2 in the early Earth atmosphere. They proposed a different composition of gases, highlighting an increased H2 level in the atmosphere, also represented in Table 1. Based on these new data, the scientists proposed an alternative graph for the changing atmospheric levels of O2 and H2O vapor, also shown in Figure 1.
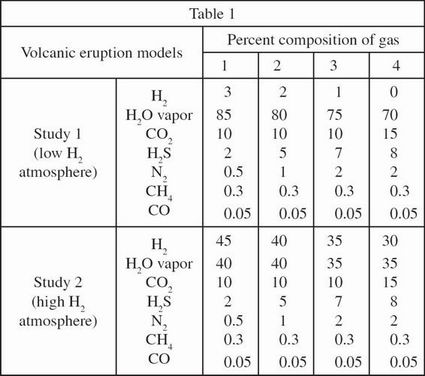
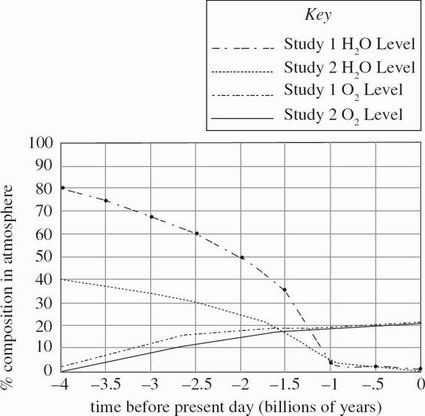
Figure 1
According to the results of Study 2, between 4 and 3 billion years before the present day, the percent composition of O2 in the atmosphere:
increased only.
increased, then decreased.
decreased only.
decreased, then increased.
According to the results of Study 1, the percent composition of H2O vapor in the atmosphere decreased most rapidly over what period of time?
Between 2.5 and 2 billion years ago
Between 2 and 1.5 billion years ago
Between 1.5 and 1 billion years ago
Between 1 and 0.5 billion years ago
Suppose that the actual early Earth atmosphere had a high H2 composition of 42%. Based on Study 2, is it likely that the corresponding H2S and N2 compositions of this atmosphere were each 3%?
3% H2S 3% N2
Yes Yes
Yes No
No Yes
No No
Suppose that in a new trial in Study 2, the percent composition of H2 in the atmosphere was set at 33%, and the percent composition of N2 was found to be 2%. The percent composition of H2O vapor in this trial would most likely be:
greater than 40%.
greater than 35% and less than 40%.
exactly 35%.
greater than 30% and less than 35%.
Consider an early Earth environment that featured microorganisms. Based on the results of Study 2, is it more likely that aerobic organisms (those that require O2 to survive) or anaerobic organisms (those that do not require O2 to survive) would have existed on Earth 4 billion years ago?
Aerobic organisms, because of the high H2
Aerobic organisms, because of the low O2
Anaerobic organisms, because of the high H2
Anaerobic organisms, because of the low O2
According to Study 2, how long did it take the H2O vapor level to decrease to 75% of its composition 4 billion years before the present day?
500 million years
1 billion years
1.5 billion years
2 billion years
Xem thêm đề thi tương tự

12 câu hỏi 1 mã đề 1 giờ
214,143 lượt xem 115,283 lượt làm bài

24 câu hỏi 1 mã đề 1 giờ
219,455 lượt xem 118,132 lượt làm bài

26 câu hỏi 1 mã đề 1 giờ
219,214 lượt xem 118,020 lượt làm bài

22 câu hỏi 1 mã đề 1 giờ
219,728 lượt xem 118,293 lượt làm bài

12 câu hỏi 1 mã đề 1 giờ
218,864 lượt xem 117,831 lượt làm bài

24 câu hỏi 1 mã đề 1 giờ
219,350 lượt xem 118,090 lượt làm bài

24 câu hỏi 1 mã đề 1 giờ
218,456 lượt xem 117,614 lượt làm bài

22 câu hỏi 1 mã đề 1 giờ
208,288 lượt xem 112,133 lượt làm bài

29 câu hỏi 1 mã đề 1 giờ
215,754 lượt xem 116,158 lượt làm bài
