
ACT Science Practice Test 6
Đề thi nằm trong bộ sưu tập: Tuyển Tập Bộ Đề Thi Đại Học Hoa Kỳ (ACT) - Có Đáp Án Chi Tiết
Số câu hỏi: 21 câuSố mã đề: 1 đềThời gian: 1 giờ
215,890 lượt xem 16,602 lượt làm bài
Xem trước nội dung:
A scientist studying hemoglobin investigated the impact of temperature and carbon dioxide (CO2) concentrations on the binding capacity of oxygen (O2). The scientist observed the binding of oxygen to hemoglobin molecules as the pressure of oxygen was increased. The temperature and CO2 were varied to identify their direct impact on the binding capacity of O2.
Figure 1 displays the impact of changes in temperature on the binding (percent of hemoglobin saturated) of oxygen. Figure 2 displays the impact of varying carbon dioxide concentrations on oxygen binding. Under normal conditions, the core body temperature is 37°C and has carbon dioxide and oxygen concentrations of 40 mmHg and 100 mmHg respectively.
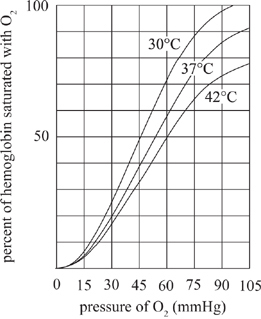
Figure 1
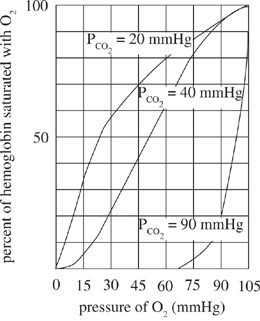
Figure 2
According to Figure 1, if the temperature is 42°C, which of the following changes in pressure of oxygen will cause the least increase in the percent of hemoglobin saturated with O2?
0-15 mmHg
15-30 mmHg
30-45 mmHg
45-60 mmHg
According to Figure 1, which of the following sets of temperature and pressure of oxygen results in the lowest hemoglobin saturation with oxygen?
Temperature (°C)
37
37
42
42
According to Figure 1, if the pressure of oxygen is 100 mmHg and 65% of hemoglobin molecules are saturated with oxygen then the core body temperature is most likely within which of the following ranges?
Less than 30°C
30°C-37°C
37°C-42°C
Greater than 42°C
Based on Figure 2, if an individual has 70% of his hemoglobin molecules saturated at a pressure of 75 mmHg of oxygen, then the individual's carbon dioxide pressure is most likely closest to which of the following?
30 mmHg
50 mmHg
70 mmHg
90 mmHg
According to Figure 2, at a CO2 pressure of 90 mmHg, as the pressure of O2 is increased from 45 mmHg to 90 mmHg, the percent of hemoglobin saturated with oxygen:
remains constant, then increases.
remains constant, then decreases.
increases, then decreases.
decreases, then increases.
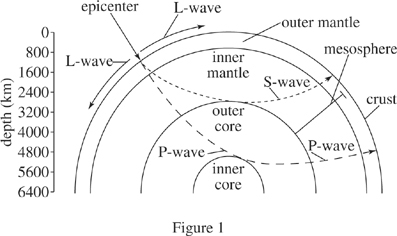
The magnitude of seismic energy released from an earthquake is often described using the logarithmic and unit-less Richter scale. Originating at the epicenter, seismic energy travels through the earth via waves such as L-waves, S-waves, and P-waves. Earthquakes with a Richter scale magnitude of 5.0 or greater can typically be detected throughout the world. Figure 1 depicts the layers of the earth and typical travel patterns of seismic waves. Table 1 lists characteristics of those seismic waves. Figure 2 shows the number of earthquakes (by magnitude) detected at a particular seismic activity monitoring station in the past 30 years, as well as the percentage probability of future earthquakes (by magnitude) in that same region in the next 30 years.
| Table 1 | ||
| Seismic wave | Depth range (km) | Crust velocity (m/s) |
| L-wave | 0-10 | 2.0-4.5 |
| S-wave | 0-2921 | 3.0-4.0 |
| P-wave | 0-5180 | 5.0-7.0 |
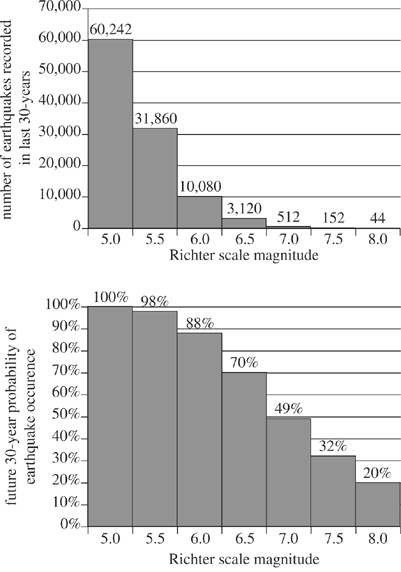
Figure 2
Figure 1 defines the mesosphere as a region of the Earth that overlaps which of the following atmospheric layers?
I.II. Inner mantle
III.II only
I and II only
II and III only
I, II, and III
A series of seismic waves was observed from an observation station. The average crust velocity of these waves was 3 m/s, and their maximum depth occurred in the inner mantle. Based on Figure 1 and Table 1, the seismic waves observed were most likely:
L-waves.
S-waves.
P-waves.
K-waves.
Given the data in Figure 2, the future probability of an earthquake occurrence decreases by more than half when comparing which of the following 2 Richter scale magnitudes?
5.0 and 6.0
6.0 and 6.5
6.5 and 7.5
7.5 and 8.0
According to Figure 2, the probability of a future earthquake occurrence is lowest for which of the following ranges of Richter scale magnitude?
5.5 to 6.0
6.0 to 6.5
6.5 to 7.0
7.0 to 7.5
Based on Figure 2, the ratio of Richter scale 5.5 earthquakes to Richter scale 5.0 earthquakes in the last 30 years can be expressed approximately by which of the following fractions?



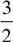
In agriculture, soils can be classified based on mineral content (the amount of various metals present in the soil), and organic content (the percent of soil volume occupied by material made by living organisms). Ideal concentrations of various minerals are given in parts per million (ppm) in Table 1. If the levels of different minerals of a soil are all similar, relative to the optimal levels, the soil is said to be well defined. If the levels of different minerals in a soil vary widely relative to the optimal levels, the soil is said to be poorly defined.
| Table 1 | |
| Mineral | Ideal concentration (ppm) |
| Nitrogen | 22 |
| Phosphorus | 14 |
| Potassium | 129 |
| Chloride | 12 |
| Sulfur | 88 |
| Iron | 6.9 |
| Manganese | 2.7 |
Study 1
Soil was taken from 5 different farms to a laboratory. The soils were desiccated (all water was removed), and a 1 L sample of each soil was prepared. In order to make sure that no minerals were trapped within the organic matter of a soil, the organic matter of each soil was burned by heating the soil to 500°C for 20 minutes. The ash of the organic matter was removed and the remaining soil analyzed for the concentration of various minerals. The results are shown, as percent of ideal concentration, in Table 2.
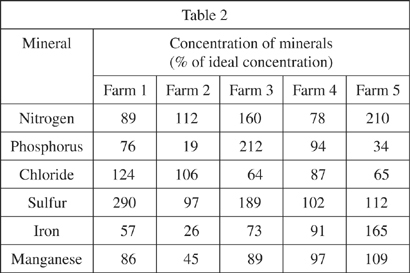
Study 2
To determine the percentage of the mass of each soil composed of organic matter, the above procedure was repeated, with the soil weighed before being heated to 500°C and after having the ash removed. The number of live cells (bacteria, fungi, etc.) in a cubic millimeter of each soil was determined by microscopic analysis. The results are presented in Table 3.
| Table 3 | ||
| Farm | % organic matter | # living cells per mm3 |
| 1 | 7.1 | 2,964 |
| 2 | 8.9 | 3,920 |
| 3 | 4.8 | 1,642 |
| 4 | 6.6 | 2,672 |
| 5 | 18.9 | 9,467 |
Soils with more living cells per mm3 generally consume more oxygen than soils with fewer living cells. Based on this information, the soil of which farm would be expected to consume the most oxygen?
Farm 1
Farm 2
Farm 3
Farm 5
If, in Study 2, before and after heating a soil sample to 500°C for 20 minutes and removing the ash, the mass of the sample was approximately the same, which of the following is the most reasonable conclusion?
There was little or no water in the soil.
There was a large quantity of water in the soil.
There was little or no organic matter in the soil.
There was little or no mineral content in the soil.
In Study 2, before heating the sample to 500°C, it was necessary for the scientists to desiccate the soil in order to ensure that:
the water was not mistaken for a mineral.
the water was not consumed by the living cells.
it was possible to count live cells by making sure the soil didn't stick together.
the mass of the water was not mistaken for organic matter.
Based on Study 2, if the scientists took a soil sample from another farm, and the number of living cells per mm3 was determined to be 2,100, the % organic matter in that soil would most likely be:
less than 4.8.
between 4.8 and 6.6.
between 6.6 and 7.1.
greater than 7.1.
Beans grow fastest in soils with high nitrogen and iron levels. If all other levels were equal, then based on the results of Study 1, which of the farms would be expected to produce the fastest growing beans?
Farm 5
Farm 4
Farm 3
Farm 2
The soil of which of the farms would likely be considered the most well defined, based on the information in Study 1?
Farm 1
Farm 2
Farm 3
Farm 4
Rock candy was made by putting a mixture of 180°°F water and an amount of sugar (S1) into an apparatus shown in Figure 1, inserting a string through the top, and allowing the mixture to stand and cool. The internal container was a jar made of glass, and the external container was made of plastic.
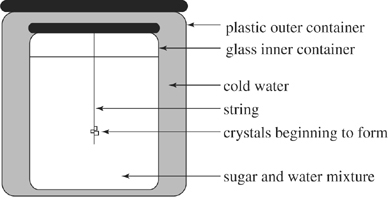
Figure 1
Figure 2 shows how the temperature of S1 and the temperature of the cold water in the outer jar varied with time as the mixture was allowed to stand.
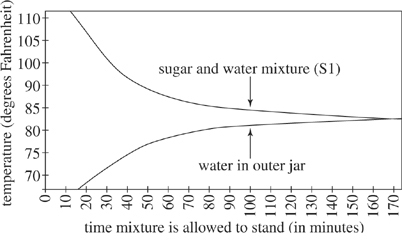
Figure 2
According to the Second Law of Thermodynamics, as the temperature of S1 decreases, the orderliness of the atoms in the solution must increase. This is why crystals form on the string, creating rock candy. Because of the Second Law of Thermodynamics, temperature of the sugar and water mixture can be monitored to measure orderliness of the atoms in the mixture. Two other sugar and water mixtures (S2 and S3) were monitored under standing conditions the same as those used for S1.
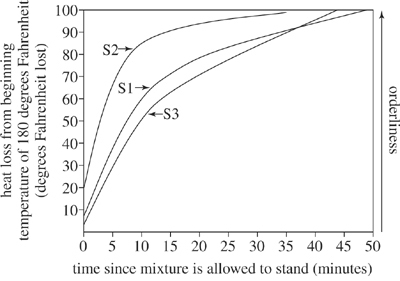
Figure 3
According to Figure 3, for S3, the heat lost from the beginning temperature of 180°F after being allowed to stand for 30 minutes is closest to which of the following?
10 degrees Fahrenheit
50 degrees Fahrenheit
80 degrees Fahrenheit
100 degrees Fahrenheit
According to Figures 2 and 3, as the temperature of the water in the outer jar increased, the heat loss from the beginning temperature of 180°F for S1:
decreased only.
increased only.
decreased, and then increased.
increased, and then decreased.
An additional sugar and water mixture (S4) was monitored under conditions identical to those used to gather the data in Figure 3. The heat lost after being allowed to stand 0 minutes was 15°F. How does the initial orderliness of the atoms in S4 compare with the orderliness of the atoms in mixtures S1, S2, and S3?
The orderliness of S4 was greater than the orderliness of S1, S2, and S3.
The orderliness of S4 was less than the orderliness of S1, S2, and S3.
The orderliness of S4 was greater than the orderliness of S2 and S3, but less than the orderliness of S1.
The orderliness of S4 was greater than the orderliness of S1 and S3, but less than the orderliness of S2.
Based on Figure 1, which of the following best explains the trends shown in Figure 2? In sum, as the time the mixture was allowed to stand increased, the heat was conducted by the:
glass jar from the sugar and water mixture to the water outside the jar.
glass jar from the water outside the jar to the sugar and water mixture.
plastic container from the string to the water outside the jar.
plastic container from the string to the sugar and water mixture.
Rock candy begins to form when the temperature of the sugar and water mixture has lost 100°F from its beginning temperature. Based on Figure 3, which mixture, if any, would begin to form rock candy first?
S1
S2
S3
All mixtures would begin to form rock candy at the same time.
