
ACT Science Practice Test 23
Đề thi nằm trong bộ sưu tập: Tuyển Tập Bộ Đề Thi Đại Học Hoa Kỳ (ACT) - Có Đáp Án Chi Tiết
Số câu hỏi: 18 câuSố mã đề: 1 đềThời gian: 1 giờ
205,935 lượt xem 15,840 lượt làm bài
Xem trước nội dung:
The boiling point of a liquid is commonly defined as the temperature at which the vapor pressure of the liquid is equal to the atmospheric pressure that surrounds the liquid. When a liquid is brought to a temperature at or above its boiling point, it quickly changes from the liquid phase to the gaseous phase. This can easily be observed in vapor bubbles that form in the liquid and rise to the top.
The boiling point of a substance depends on many different factors, such as the composition of the substance, its molar mass, and the atmospheric pressure surrounding it. The names, molar masses, and skeletal models of a variety of alkanes and three alcohols were identified and recorded in Table 7.2.
TABLE 7.2
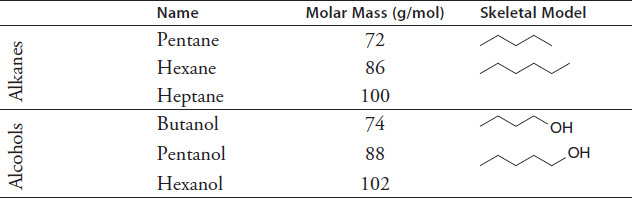
Figure 7.4 shows the boiling points of these six substances versus their molar mass. All temperatures are in degrees Celsius and have been recorded at a standard atmospheric pressure of 1 atm.
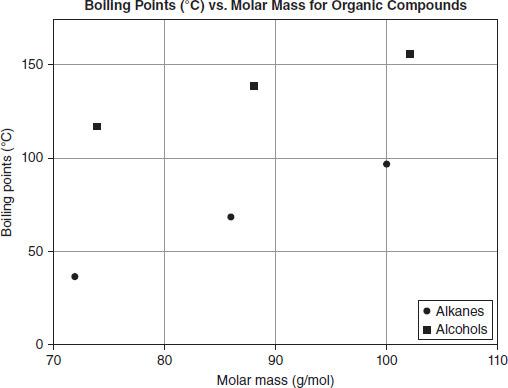
Figure 7.4
What is the boiling point of heptane?
37°C
97°C
117°C
156°C
Based on Figure 7.4, what is the best summary of the relationship between the molar mass and boiling point of an alkane?
The molar mass of a substance seems to have little effect on its boiling point.
As the molar mass of a substance increases, its boiling point tends to increase.
As the molar mass of a substance increases, its boiling point tends to decrease.
The relationship between the molar mass and boiling point of a substance is not clear from the data in the figure.
The organic substance propanol has a molar mass of about 60 g/mol. Which temperature is most likely the boiling point of propanol?
11°C
52°C
97°C
117°C
In which physical state would pentanol be if the atmospheric pressure were 1.0 atm and the temperature were 150°C?
Solid
Liquid
Gaseous
Cannot be determined
Based on the skeletal models shown in Table 7.2, which statement best describes the effect of a hydroxyl group (OH-) on the boiling point of a substance?
When the molar mass of a substance is controlled, the addition of a hydroxyl group tends to increase the substance's boiling point.
When the molar mass of a substance is controlled, the addition of a hydroxyl group tends to decrease the substance's boiling point.
When the molar mass of a substance is controlled, the addition of a hydroxyl group tends to have no effect on the substance's boiling point.
The figure does not show a relationship between the presence of a hydroxyl group and the boiling point of a substance.
Based on the skeletal models shown in Table 7.2, which of the following images shows the skeletal model for hexanol?




Organic chemists draw skeletal models to simplify the actual atoms and connections that exist within a molecule. Each short line segment represents the connection between two carbon atoms. In alkane molecules, hydrogen atoms surround each carbon atom in such a way that there are three hydrogen atoms on each end carbon and two hydrogen atoms on each middle carbon. These molecules can also be represented by ball-and-stick models or chemical formulas. An example for the molecule hexane is shown in Figure 7.5.
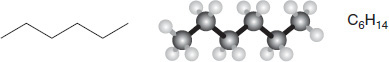
Figure 7.5
The alkane molecule, decane, has the following skeletal structure.

What is the chemical formula for decane?
C1020
C1022
C1024
C1030
Decane has a molar mass of 142 g/mol. What could a person expect its boiling point to be?
212°C
185°C
156°C
142°C
The sun is a source of many wavelengths of radiation that reach the earth. The earth's atmosphere absorbs some of these wavelengths, while others are able to penetrate and reach the planet's surface. Ultraviolet radiation from the sun comes in three different categories based on wavelength and penetration: UVA, UVB, and UVC.
UVB radiation has wavelengths of 280 to 320 nm and is partially absorbed by the earth's ozone layer. The UVB rays that do reach the surface can be absorbed by human skin and have been known to cause sunburn and many forms of skin cancer. Many products, from glasses to sunscreen, have been created to help protect humans from UVB radiation. Two groups of students set out to test the ability of materials to block UVB light, using a computer and a sensor specifically designed to detect UVB radiation.
Group 1
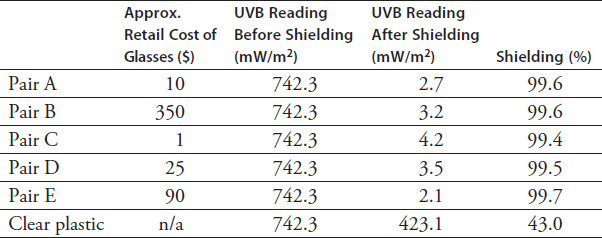
The members of Group 1 placed a sensor in full sunlight and shielded the sensor with a variety of sunglasses claiming to offer UVB protection. A reading was taken on the UVB sensor for each product, and the data were recorded in Table 8.1.
TABLE 8.1
Group 2
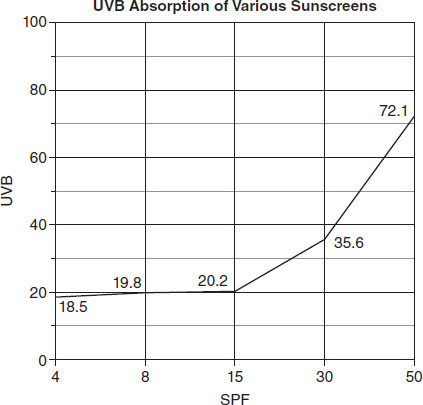
The members of Group 2 placed a sensor in the sun and shielded that sensor with a piece of glass. They tested sunscreens of increasing SPF (sun protection factor) on the glass, and the data were recorded in Table 8.2.
TABLE 8.2
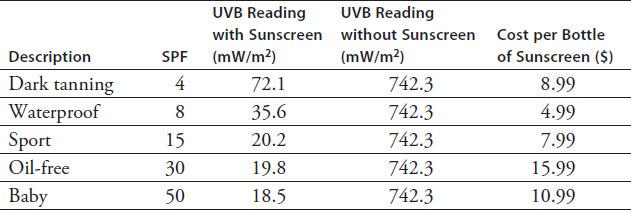
In Group 2's experiment, SPF is the independent variable being manipulated and UVB is the dependent variable being measured. Which of the following graphs best represents the relationship between the SPF and UVB data from this experiment?
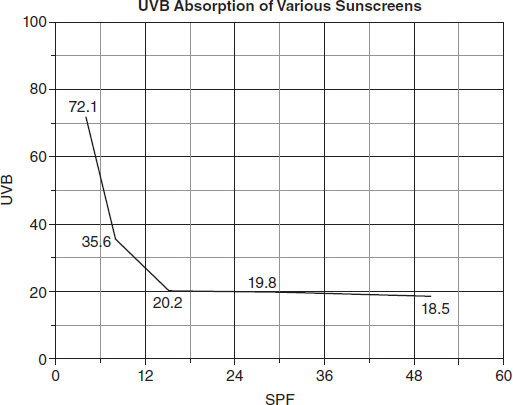
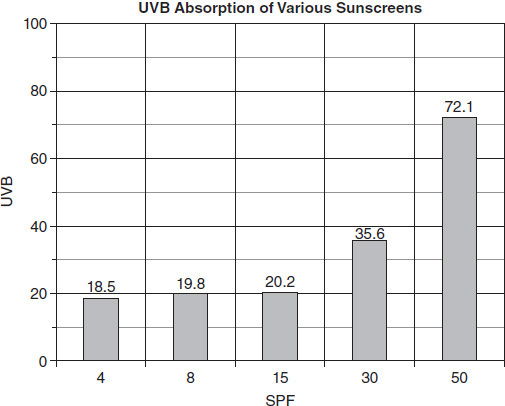
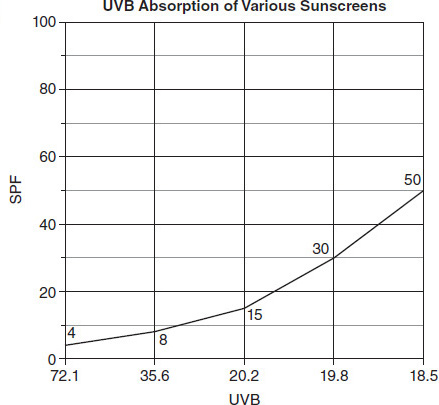
Which of the following questions could the students in Group 2 be attempting to answer using the data from their experiment?
Does the cost of sunscreen affect the amount of UVB that is blocked?
Which brand of sunscreen is the best?
Is there a relationship between SPF and the amount of UVA radiation blocked?
Can sunscreen protect humans from skin cancer?
According to the findings of Group 1:
there is a negative correlation between price and the ability to protect from UVB radiation.
there is a positive correlation between price and the ability to protect from UVB radiation.
there is no correlation between the price of a pair of sunglasses and their ability to protect the eyes from UVB radiation.
sunglasses do not protect the eyes from UVB radiation.
Assuming that the sunscreen being tested was purchased in 10-oz bottles, which sunscreen had the best cost for the amount of UVB protection (use the formula $/mW/m2 of UVB blocked)?
SPF 50
SPF 30
SPF 8
SPF 4
Which of the following is MOST likely to represent a control that would have been used to ensure reliability of data in the experiment done by Group 2?
The distance from the sunglasses to the sensor
The ingredients in the sunscreen being used
A longer time for the sensor readings as SPF increased
The amount and thickness of sunscreen being spread on the glass
According to the results of Group 1's experiment, what percentage of UVB rays would a $200 pair of sunglasses block?
99.50%
99.70%
99.60%
There is not enough information to determine this answer.
Based on the results of Group 2's experiment, what would the UVB reading most likely be if SPF 60 sunscreen were to be tested?
18 mW/m2
19.25 mW/m2
9.25 mW/m2
4.5 mW/m2
Which of the following is the experimental variable that Group 1 manipulated?
The time of day
The type of sunglasses
The amount of UVB rays
The distance of the materials from the sensor
Which of the following implies the correct relationship between SPF and UVB blockage?
Sunscreens with SPFs higher than 30 provide only a marginal increase in sun protection over their counterparts with lower SPFs.
As SPF increases, the ability to block UVB light decreases.
There is no correlation between SPF and UVB blockage.
Sunscreens with higher SPFs provide less sun protection than those with low SPFs.
What other experiment could the students in Group 2 conduct using the same equipment?
The effect of sunscreen on shielding UVA rays
The ability of sunscreen to prevent cancer
How the amount of sunscreen applied can impact the SPF
The margin of benefit of SPF sunscreens higher than 60
