
ACT Science Practice Test 32
Đề thi nằm trong bộ sưu tập: Tuyển Tập Bộ Đề Thi Đại Học Hoa Kỳ (ACT) - Có Đáp Án Chi Tiết
Số câu hỏi: 22 câuSố mã đề: 1 đềThời gian: 1 giờ
193,447 lượt xem 14,877 lượt làm bài
Xem trước nội dung:
In 1922, Niels Bohr revised the atomic model to include a positively charged nucleus surrounded by negatively charged electrons that traveled in well-defined shells around the nucleus. The shells can be thought of as concentric circles around the nucleus. A neutral atom contains the same number of protons in the nucleus as electrons surrounding the nucleus. The inside shell can hold two electrons, and the second shell can hold eight electrons. An electrostatic attraction occurs between the positively charged protons in the nucleus and the negatively charged electrons. A representation of Bohr's shell model is shown in Figure 12.1.
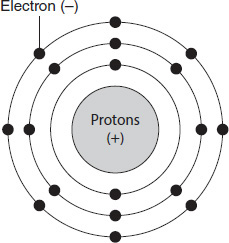
Figure 12.1
Partial evidence for this atomic shell model comes from the study of ionization energies of different elements. Ionization energy is the amount of energy required to remove an electron from an atom or ion in the gaseous state. The first ionization energy removes the electron farthest from the nucleus and can be represented by the following formula:
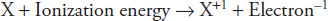
where X represents a neutral atom.
The nth ionization energy removes additional electrons from an already charged ion. For example, the third ionization energy can be represented by the following formula:
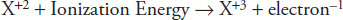
The ionization energies in kJ/mol of the first 10 elements can be found in Table 12.1.
TABLE 12.1
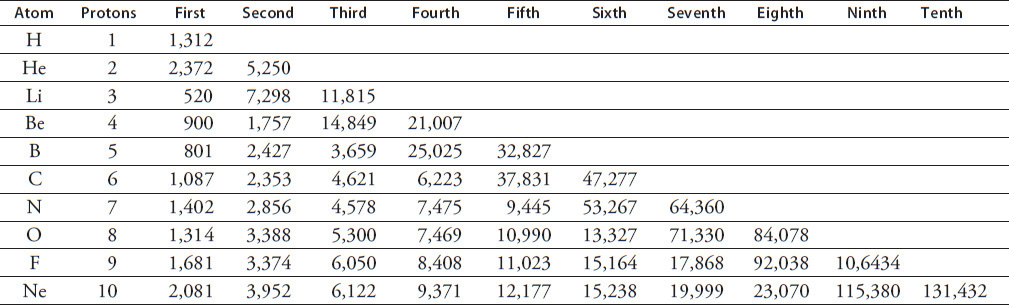
How much energy does it take to remove the outermost electron from beryllium (Be)?
900 kJ/mol
1,757 kJ/mol
14,849 kJ/mol
21,007 kJ/mol
Which of the following statements regarding the ionization energy of the third proton of the elements listed is true?
There is a general and consistent increase as the elements get larger.
There is a general and consistent decrease as the elements get larger.
Increases are then followed by a decrease.
After an early dip, there is a general increase.
How much energy is required to remove an electron from nitrogen as shown in the following equation?
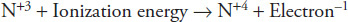
1,402 kJ/mol
2,856 kJ/mol
4,578 kJ/mol
7,475 kJ/mol
The first ionization energy of Element 2, hydrogen, is much larger than the first ionization energy for Element 3, lithium. Which statement best explains this trend?
Helium has only two electrons while lithium has three.
Helium is a very light element; therefore, it is very hard to remove its electrons.
Both of helium's electrons are the first shell, while one of lithium's electrons is found in the second shell.
Helium has two protons providing the positive electrostatic attraction, while lithium has three protons providing a larger attraction.
Which best explains why there is no seventh ionization energy listed for the element carbon?
It is hard to measure the energy required to remove carbon's seventh electron.
Carbon does not have seven electrons surrounding its nucleus.
The seventh ionization energy of carbon is equal to the first ionization energy of nitrogen, so it does not need to be listed.
The seventh, eighth, ninth, and tenth ionization energies of carbon are all equal to the sixth, so they do not need to be listed.
Figure 12.2 shows an electron being removed from the element oxygen. How much energy is associated with the image shown?
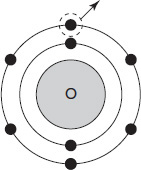
Figure 12.2
1,314 kJ/mol
3,388 kJ/mol
71,330 kJ/mol
84,078 kJ/mol
According to Table 12.1, how many electrons must the element nitrogen have in its outermost shell?
Two
Five
Seven
Eight
Referring to Table 12.1, what evidence supports the fact that each element has only two electrons in the first, innermost shell?
The first two ionization energies are always smaller than the rest.
The last two ionization energies are always significantly larger than the rest.
There is a significant jump between the second and third ionization energies for most elements.
The first two ionization energies of helium and lithium are both relatively small.
Which set of ionization energy (IE) data represents an atom that has one electron in its outermost electron shell?
First IE, Second IE, Third IE, Fourth IE
300, 600, 1801, 2230
700, 2892, 3019, 3299
1121, 1398, 4456, 4876
854, 1981, 2765, 3344
Helium and lithium are isoelectronic. This means their electron structure is identical. They both have two electrons in the first shell outside of the nucleus. Why does it take less energy to remove an electron from helium than it does to remove an electron from lithium?
Helium has two protons, but lithium has three.
Lithium has already had the outermost electron removed.
The first ionization energy of lithium is smaller than that of helium.
Lithium has a positive charge so removing electrons is more difficult.
How many kJ of energy would be required to remove all of the electrons from 1 mol of helium atoms?
2,372 kJ
5,250 kJ
7,622 kJ
10,500 kJ
Many companies advertise that their brand of battery outlasts the competitors' batteries. A series of experiments were conducted to compare batteries from different manufacturers. Figure 12.3 shows the results of tests of four different brands of batteries. Two AA batteries were tested in an incandescent bulb flashlight, and the combined voltage was tested for continuous use over time. The flashlight operated effectively for voltage values greater than 2.2 V.
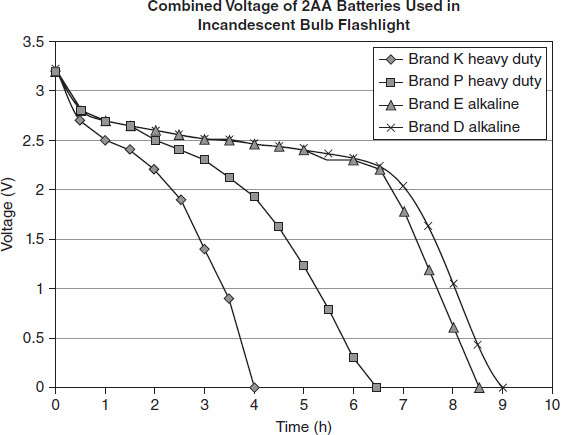
Figure 12.3
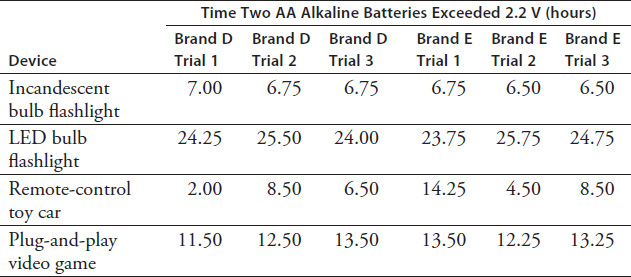
Table 12.2 compares how Brands D and E alkaline batteries performed in different devices. The table displays the time the combined voltage of two batteries remained above 2.2 V. The voltages were checked every quarter-hour.
TABLE 12.2
According to Figure 12.3, how did the performance of Brands D and E alkaline batteries compare?
Brand E performed significantly better than Brand
Brand D performed significantly better than Brand
There was no significant difference between the performance of the brands.
Neither Brand D nor Brand E was capable of lighting the flashlight for more than an hour.
According to Figure 12.3, the Brand P battery was capable of operating the incandescent flashlight for approximately:
0.5 hours.
2.0 hours.
3.5 hours.
6.5 hours.
Which of the following conclusions is plausible from the information provided in Figure 12.3?
Heavy-duty batteries perform better than alkaline batteries when used in medium-drain devices such as incandescent bulb flashlights.
In the experiment, there was more consistency in the performance of alkaline batteries than in that of heavy-duty batteries.
Alkaline batteries perform better than heavy-duty batteries in high-drain devices such as camera flashes.
The Brand P heavy-duty battery is the best value for the money spent.
What can one conclude about the performance of Brands D and E batteries when they were used in the remote-control car?
Brand E performed significantly better than Brand
Brand D performed significantly better than Brand
The two brand's performances were approximately the same.
The variability of the data prevents any valid comparison.
When used in the incandescent bulb flashlight, how much time did Brand K's heavy-duty battery sustain a voltage between 2 and 3 V?
0.5 hours
2.0 hours
3.0 hours
6.5 hours
Using the data in Table 12.2, how do the averages of the three trials compare when Brands D and E are used in the plug-and-play video game?
Brand D's average was 0.75 hours more than Brand E's.
Brand E's average was 0.5 hours more than Brand D's.
Brand E's average was 1.5 hours more than Brand D's.
Brands E and D had the same average.
Which of the following statements is true according to Figure 12.3?
In the first few hours of testing, the voltage drop for Brand D was greater than that for Brand
Brand P's performance over the first four hours was nearly identical to that of Brand
At voltages under 2.2 V, the voltage of Brand E drops more rapidly than that of Brand
Brand K lasts longer than Brand
Which of the following steps in the experimental procedure is MOST IMPORTANT in the comparison of battery performance between brands?
The batteries tested were manufactured in the same year.
The batteries tested were not previously used in any other device.
The temperature was controlled in the testing room.
The time intervals for measuring voltages were the same for each brand.
Which of the following devices is most likely classified as a low-drain device?
Incandescent bulb flashlight
LED bulb flashlight
Remote-control toy car
Plug-and-play video game
Approximately how much longer than the incandescent bulb flashlight does the LED bulb flashlight last?
Twice as long
Four times as long
Six times as long
Twenty-four times as long
In terms of just the Brand E tests, which of the following devices is most likely classified as a high-drain device?
Incandescent bulb flashlight
LED bulb flashlight
Remote-control toy car
Plug-and-play video game
