
ACT Science Practice Test 33
Đề thi nằm trong bộ sưu tập: Tuyển Tập Bộ Đề Thi Đại Học Hoa Kỳ (ACT) - Có Đáp Án Chi Tiết
Số câu hỏi: 22 câuSố mã đề: 1 đềThời gian: 1 giờ
211,223 lượt xem 16,246 lượt làm bài
Xem trước nội dung:
Astronomers have found over 400 planets orbiting stars. The discovered planets have a variety of compositions, masses, and orbits. Despite the variety, the universal rules of physics and chemistry allow scientists to broadly categorize these planets into just a few types: Gas Giant, Carbon Orb, Water World, and Rocky Earth. Table 1 shows the composition of the various planet types and typical mass ranges relative to Earth.
Table 1
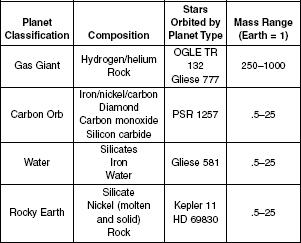
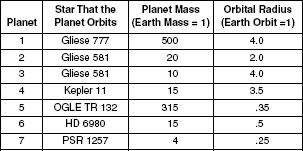
Table 2 shows a sampling of planets orbiting various stars described in Table 1. These planets are merely numbered 1–7. Table 2 details the masses and orbital radii of the planets.
Table 2
The data in Table 1 and Table 2 support which of the following statements?
Gas Giant planets have the largest orbital radii.
Orbital radius is directly related to mass.
Orbital radius is inversely related to mass.
The data does not support a correlation between mass and orbital radius.
According to Table 1 and Table 2, which of the following stars has the most massive Gas Giant planet orbiting it?
Gliese 777
OGLE TR 132
PSR 1257
Gliese 581
If a new planet were discovered, with a mass of 325, an orbital radius of 1.5, and a composition of mostly hydrogen, what would be its most likely classification?
Carbon Orb
Water
Rocky Earth
Gas Giant
All of the following are true about the star Gliese 581 EXCEPT:
Gliese 581 has multiple planets orbiting it.
Gliese 581 has the most massive planet orbiting it.
Gliese 581 has planets orbiting it that are classified as water.
Gliese 581 has only planets with masses less than 50 Earth masses orbiting it.
The Rocky Earth planets in Table 2 have what in common?
Their masses are less than 20 Earth masses.
They are only found in deep space.
Hydrogen is a major component of the crust.
Their orbits are all less than 1 Earth radius.
A group of researchers studied the greenhouse gas (GHG) and energy savings associated with rigid plastic foam sheathing used to insulate single-family housing. The results show the typical annual energy savings for a single house in the United States. Table 1 displays energy savings for various temperature zones, whereas Table 2 shows GHGs avoided.
Table 1
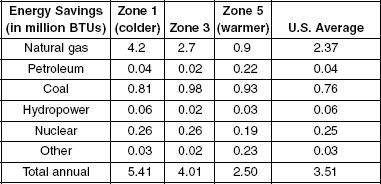
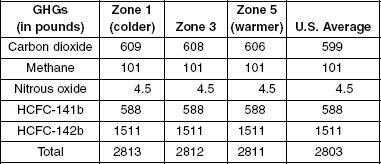
Table 2
There is an initial use of energy and GHG released when the insulation is installed, but large savings result from the use of the product over time. After the “payback” time expires, there is a net savings in energy and GHG emissions for as long as the insulation is in place. Figure 1 displays the payback time in years for energy savings and GHGs avoided.
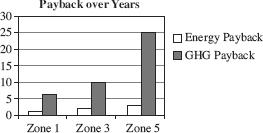
Figure 1
Foam sheathing proved to create the highest energy savings in Zone 5 for what type of energy?
Coal
Nuclear
Natural Gas
Petroleum
According to the data presented in Table 1 and Table 2, which of the following is true?
Foam sheathing prevents more HCFC-141b than HCFC-142b from entering the atmosphere.
The yearly energy savings vary more by zone than the yearly GHGs avoided.
The warmer the zone, the less the energy savings from petroleum.
The average for GHGs avoided is greatest for carbon dioxide.
According to Figure 1, the GHG payback for Zone 3 is approximately:
11 years.
2 years.
13 years.
9 years.
According to Table 1, what zone and fuel combination yield the greatest energy savings after 1 year?
Nuclear in Zone 1
HCFC-142b in Zone 3
Coal in Zone 5
Natural gas in Zone 1
Ten years after installing rigid plastic foam sheathing in Zone 3, which of the following is true?
The customer will have produced fewer GHGs, but will not yet have saved money on energy.
The customer will have saved money on energy, but will not yet have produced fewer GHGs.
The customer will have neither saved money on energy nor produced fewer GHGs.
The customer will have both saved money on energy and produced fewer GHGs.
Based on the data in Table 1, which of the following lists the fuels that yielded the highest average energy savings, in decreasing order?
Natural gas, coal, and nuclear
Hydropower, nuclear, and other
Coal, natural gas, and nuclear
Nuclear, petroleum, and coal
Ohm’s law states that V = I × R and WRC/L where V = voltage, I = current, R = resistance, C = cross-sectional area of the wire, W = resistivity of the wire, and L = length of the wire. Using the circuit pictured in Figure 1, a student performed two experiments.
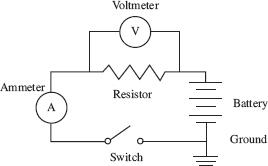
Figure 1
Experiment 1
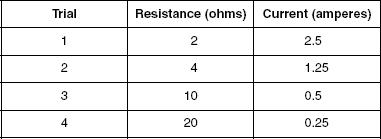
A 5 V battery was used and the resistance was varied. Table 1 displays the results.
Table 1
Experiment 2
A battery of 1 volt was used and three different wires, each with the same resistivity and length, were used to complete the circuit. Table 2 shows the results and Figure 2 shows the relationship between the diameter of the wire and the measured resistance of each wire.
Table 2
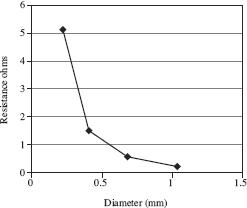
Figure 2
In Table 1, what is true about the relationship between current and resistance?
As resistance increased, current increased.
As resistance increased, current was unchanged.
As resistance increased, current decreased.
There is no relationship between current and resistance.
Based on Table 2 and Figure 1, it could be determined that a wire with a diameter of .5 mm would have a resistance close to:
2.6 ohms.
1.0 ohms.
.5 ohms.
.25 ohms.
In Experiment 2, which of the following factors was varied?
Voltage of the battery
Diameter of the wire
Length of the wire
The material of the wire
The following hypothesis was put forth prior to the experiments: Current is inversely related to resistance. An inverse relationship implies that when one quantity increases, the other decreases. Do Tables 1 and 2 support this hypothesis?
No. Experiment 1 contradicts the hypothesis and shows a direct relationship between current and resistance. The current increases with the resistance.
No. Experiment 2 contradicts the hypothesis and shows a direct relationship between current and resistance. The current increases with resistance.
Yes. The results of both experiments show an inverse relationship between current and resistance. When the resistance increases, current decreases.
No. Neither experiment shows any relationship between current and resistance.
Using the circuit in Figure 1 and the results of both experiments, which of the following conditions would result in the largest current?
5-volt battery, .5-mm wire
5-volt battery, .25-mm wire
2.5-volt battery, .5-mm wire
2-volt battery, .25-mm wire
Based on the information in the passage, figures, and tables, which of the following sets of conditions would most likely produce a current of about 5 amperes (assume the wire used is the same wire in Experiment 2)?
Voltage: 5 volts; wire diameter: .7 mm
Voltage: 10 volts; wire diameter: 1 mm
Voltage: 1 volt; wire diameter: .2 mm
None of the above conditions would produce a current near 5 amperes.
Scientists tested a new method of titration, called atomic absorption inhibition titration (AAIT). They ran a series of experiments to determine if AAIT could be used successfully to determine the presence of phosphate, silicate, and sulfate in river and waste water. AAIT involves continually adding magnesium to a stirred sample solution while monitoring the solution for magnesium absorption.
Experiment 1
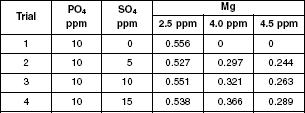
The scientists conducted an experiment to determine the effect of sulfate on titration of phosphate. Four trials were conducted, varying the concentration of both the sulfate and the magnesium used for the titration. Table 1 displays the results of the AAIT for solutions containing phosphate and sulfate and varying concentrations of magnesium.
Table 1
Experiment 2
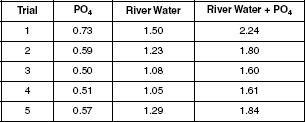
Analysis of water from the Milwaukee River was performed. Solutions containing phosphate and sulfate were analyzed using AAIT. Titrations were performed on river water, river water plus the addition of phosphate, and standardized phosphate solution. The endpoint was noted for each trial when the titration reached the conditions under which only silicate would be detected. Table 2 shows the data collected, in ml.
Table 2
Experiment 3
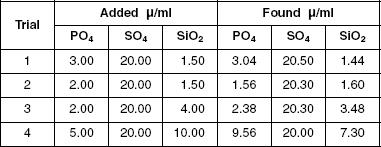
Scientists created artificial waste water by adding phosphate, silicate, and sulfate to water and then conducting AAIT to simultaneously determine how much of each substance was in the water. Four titrations were conducted. Table 3 displays the results.
Table 3
According to the data in Table 2, which trial resulted in the most silicate being detected in river water?
Trial 1
Trial 2
Trial 3
Trial 4
Based on the information presented in Table 3, which trial resulted in less PO4 being found than was initially added?
Trial 1
Trial 2
Trial 3
Trial 4
Before conducting Experiment 1, the scientists hypothesized that the higher the concentration of Mg used, the more Mg that would be absorbed. Do the results of the experiment support the hypothesis?
Yes. For each trial as the concentration of Mg used increased from 2.5 to 4.5 ppm, more Mg was absorbed.
Yes. Table 1 shows that the most Mg absorbed was during Trial 1 and with an Mg concentration of 2.5 ppm.
No. The opposite has been shown to be the case. For each trial as the Mg concentration increased, the amount of Mg absorbed decreased.
No. There is not a clear relationship between the Mg concentration and the amount of Mg absorbed.
Based on the results of Experiment 2, what type of solution was found to reach the endpoint with the least amount of solution?
Phosphate and sulfate
Standardized phosphate solution
River water
River water plus phosphate
The data in Table 1 supports which of the following statements?
The titrations done using Mg with a concentration of 4.0 ppm resulted in the most Mg being absorbed.
The titrations done using Mg with a concentration of 2.5 ppm resulted in the least Mg being absorbed.
With a Mg concentration of 4.5 ppm, as the titrations included more concentrated SO4, less Mg was absorbed.
With a Mg concentration of 4.5 ppm, as the titrations included more concentrated SO4, more Mg was absorbed.
