
ACT Science Practice Test 37
Đề thi nằm trong bộ sưu tập: Tuyển Tập Bộ Đề Thi Đại Học Hoa Kỳ (ACT) - Có Đáp Án Chi Tiết
Số câu hỏi: 22 câuSố mã đề: 1 đềThời gian: 1 giờ
205,334 lượt xem 15,790 lượt làm bài
Xem trước nội dung:
Students crystallized an impure, solid compound. First, they added just enough hot solvent to dissolve the compound. They then allowed the hot solution to cool, whereupon crystals began to form. Finally, the solution was placed in an ice bath to complete the crystallization process (see Figures 1 and 2). Figure 3 illustrates the progression of crystallization.
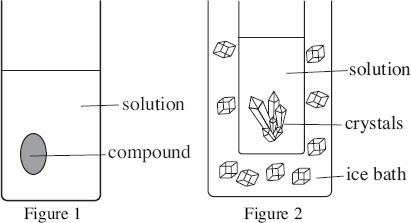
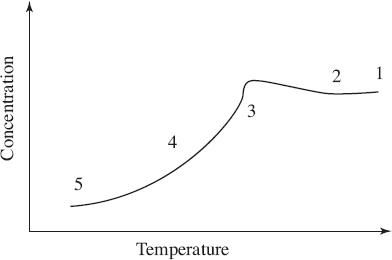
1 Solution added, undersaturated
2 Solution cools to saturation
3 Concentration decreases with crystal growth
4 Crystal growth during main cooling cycle
5 Supersaturated
Figure 3
Solubility (the amount of solute that will dissolve in a specific solvent) and crystallization are directly related. Since crystallization cannot begin until the solution becomes saturated, the faster a compound dissolves, the more quickly it can begin to form into crystals.
Study 1
Students tested the solubility of four different substances. The temperature was measured in °C, and water was used as the solvent. The results of the study are displayed in Figure 4.
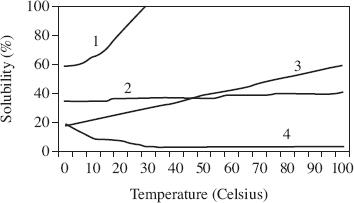
Figure 4
In a solution of 60°C water, which sample from Study 1 would begin the crystallization process first?
Sample 1
Sample 2
Sample 3
Sample 4
A fifth sample was tested for solubility under the same conditions as in Study 1. The solubility at 30°C was 20%. How did the solubility of Sample 5 compare with that of Samples 1–4?
It was lower than Samples 1–4.
It was higher than Samples 1–4.
It was lower than Samples 3 and 4 but higher than Samples 1 and 2.
It was lower than Samples 1–3 but higher than Sample 4.
According to Figure 4, the solubility of Sample 1 at a water temperature of 30°C was closest to which of the following?
20%
60%
80%
95%
Based on the information in the passage and Figure 3, the solution was most likely placed in an ice bath at which of the following points?
1
2
4
5
Based on Figure 3, which of the following best explains the relationship between temperature and crystallization?
As temperature decreases, crystallization increases.
As temperature increases, crystallization increases.
As temperature decreases, crystallization decreases.
Temperature and crystallization do not affect one another.
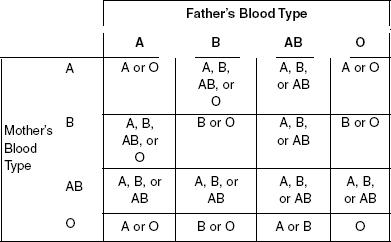
Blood type is a hereditary trait. The type is established by the genes inherited from the mother and father. The ABO system is widely accepted as the best blood classification system. In the ABO system, there are four types of blood: A, B, AB, and O. The combination of inherited genes is known as the genotype and the actual blood type is known as the phenotype. The genes ensure that only the blood cells of the proper blood type remain in the body.
Table 1
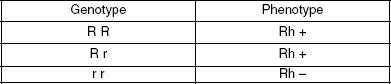
The Rh (+/–) factor is inherited separately from the blood type. It is possible to have the Rh+ phenotype yet still carry the recessive gene for Rh–. The Rh+ (R) is the dominant gene and Rh– (r) is recessive. Table 2 shows the Rh phenotypes resulting from the various genotypes inherited from parents.
Table 2
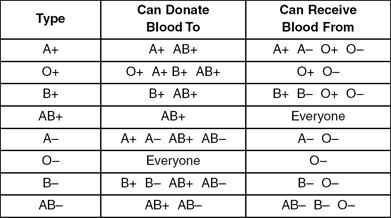
The surface of every red blood cell is covered with proteins. Rh factor and blood type determine the proteins and the compatibility of donated blood as shown in Table 3.
Table 3
According to Table 1, parents with blood types O and B can only produce offspring with which blood types?
B or O
A or B
A or O
A
Parents with which blood types could produce offspring with AB+ blood?
O+ and A–
A– and B+
AB– and AB–
B+ and B+
A person who can donate blood to anyone could have parents with which of the following blood types?
A+ and AB–
AB– and AB–
B– and B–
AB+ and AB+
List all of the blood types possible for the offspring of parents of blood types A+ and O+.
A+ or O+
A+, A–, O–, or O+
O– or O+
AB+, A–, or O–
The genes that determine blood type are also responsible for:
Rh factor.
controlling the types of cells in the blood.
controlling blood volume.
creating proteins on white blood cells.
In a study of the effects of Ritalin and Adderall on children with ADHD, subjects were given one of four possible doses of medication. Their behavior in social and academic settings was then monitored and rated. The four possible doses were placebo (P), Ritalin given once in the morning (R1), Ritalin given twice daily (R2), or Adderall given once in the morning (A1).
The results for each group were averaged. Figure 1 shows the average behavioral rating (on a scale of 0–15, with 0 meaning no undesirable behavior) at various time periods throughout the day. Figure 2 shows the percentage of children who demonstrated side effects at a moderate or severe level on at least one day.
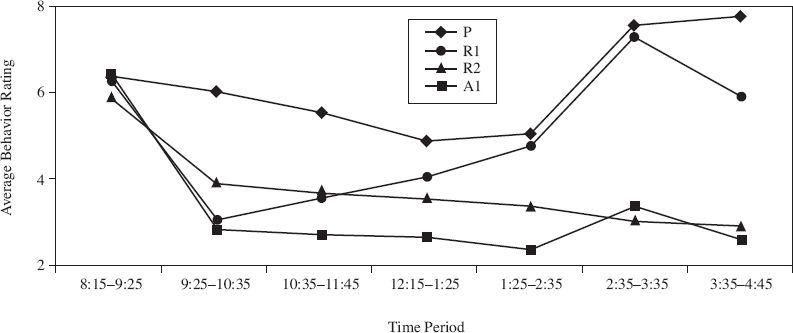
Figure 1
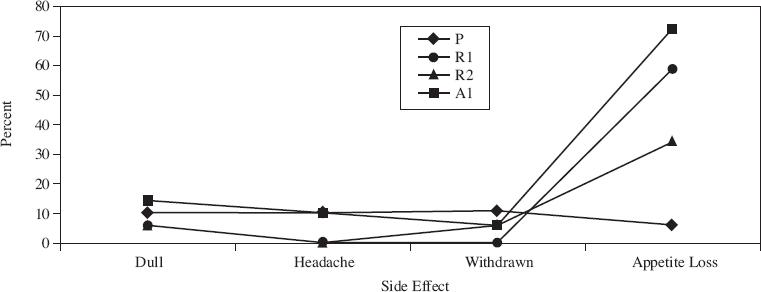
Figure 2
Based on Figure 1, during which of the following time periods was the average behavior rating most similar for the four groups of children?
8:15–9:25
9:25–10:35
10:35–11:45
3:35–4:45
A scientist claimed that children given one dose of Adderall daily would exhibit fewer behavior problems than children given either one or two doses of Ritalin daily. During which of the following time periods shown in Figure 1 are the results inconsistent with this claim?
9:25–10:35
10:35–11:45
2:35–3:35
3:35–4:45
According to Figure 2, for the group given Ritalin twice daily, the percentage of children who experienced an adverse side effect was greatest for which side effect?
Dull
Headache
Withdrawn
Appetite loss
According to Figure 1, which dose of medication was the least successful in controlling children’s behavior problems from 3:35–4:45?
P
R1
R2
A1
Suppose four groups of children were given one of the four possible medication regimens. Between 12:15 and 1:25, one group had a behavior rating of 5. Which medication regimen was most likely given to them?
P
R1
R2
A1
Assume that an ideal medication is one that has the fewest side effects, yet is most effective. Based on the data provided, which dose of medication is the most ideal?
P
R1
R2
A1
A solution consists of a solute dissolved in a solvent. For example, in a saltwater solution, the salt is the solute and the water is the solvent. Osmosis is the movement of solvent molecules across a barrier in order to equalize solution concentrations. When a saltwater solution is placed in a u-shaped tube with a selectively permeable barrier or semipermeable membrane, the phenomenon of osmotic pressure can be observed. Osmotic pressure is the pressure required to prevent the flow of a solvent across a barrier. In Figure 1, saltwater was poured in the right half of the tube and pure water was poured in the left half of the tube. As water passes from left to right in the figure below due to osmosis, the level of solution on the right rises. Eventually, the weight of the solution due to gravity becomes sufficient to prevent the further flow of water from right to left.
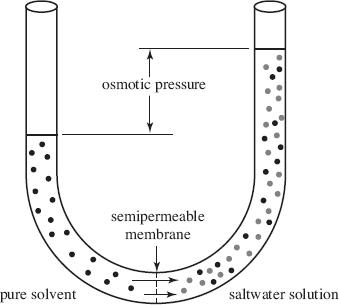
Figure 1
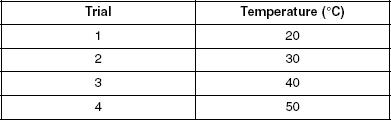
The following experiments were carried out to study how varying the molecular weight percentage of solvents and the temperature of solutions affects osmotic pressure. Table 1 shows the molecular weight percentages of the different solvents studied and Table 2 displays the temperatures of the various solutions used.
Table 1
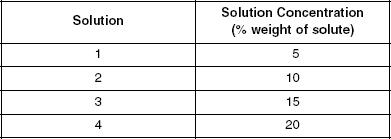
Table 2
Experiment 1
A 1000 ml u-shaped tube was fitted with a semipermeable membrane and 300 ml of solution was added on the right half and 300 ml of water was added on the left half. Table 1 shows the four solutions used in four separate trials of Experiment 1. The percent weight was measured by computing the ratio of the weight of the solute (salt) to the weight of the solution after dissolution. Every 5 seconds, the height of the solution on the right side of the tube was measured and recorded. Figure 2 shows the results of Experiment 1 recorded at 20°C.
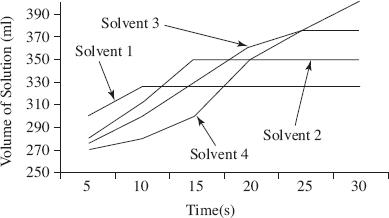
Figure 2
Experiment 2
Four more trials were performed but only the 10% solution was used. For each trial, the temperature was varied as shown in Table 2 and the results were graphed in Figure 3. In each trial, the height was measured 5 minutes after the water and solution were poured.
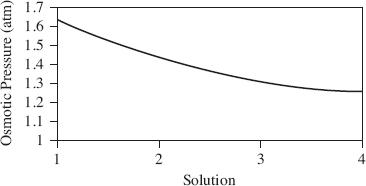
Figure 3
Based on Figure 2 and Table 2, which solution resulted in the greatest osmotic pressure after 30 seconds?
Solution 1
Solution 2
Solution 3
Solution 4
Based on the results of Experiment 2, what is the relationship between osmotic pressure and temperature?
Osmotic pressure increases with an increase in temperature.
Temperature and osmotic pressure are unrelated.
Osmotic pressure decreases with an increase in temperature.
Five minutes was insufficient time to allow the effects of osmotic pressure to be observed.
Based on Experiment 1, how does solution concentration affect osmotic pressure?
Osmotic pressure increases with concentration.
Osmotic pressure decreases with concentration.
There is no relationship between osmotic pressure and concentration.
The results were inconclusive because all solution heights were still fluctuating after 30 seconds.
Was Experiment 1 successful in determining the relative osmotic pressure of the four solutions tested?
Yes. There is a clear difference in osmotic pressure between the four solutions with Solution 1 having the greatest osmotic pressure.
Yes. There is a clear difference in osmotic pressure between the four solutions with Solution 4 having the greatest osmotic pressure.
No. All osmotic pressures were too similar to make any determination.
No. All solution heights were still fluctuating after 30 seconds.
Reverse osmosis occurs when the solvent flows from a solution of higher concentration to a solution of lower concentration. In Experiments 1 and 2, reverse osmosis can be observed by pouring more solution in the right side of the tube. Assuming the pressure required for reverse osmosis occurs at twice the osmotic pressure, approximately what volume of the 30% solution in Experiment 1 would have to be added for reverse osmosis to occur?
0.7 atm
1.2 atm
2.8 atm
3.2 atm
Data in Figure 2 is consistent with which of the following trials?
Trial 1 and Trial 3
Trial 1 only
Trial 2 only
Trial 2, Trial 3, and Trial 4
