
ACT Science Practice Test 39
Đề thi nằm trong bộ sưu tập: Tuyển Tập Bộ Đề Thi Đại Học Hoa Kỳ (ACT) - Có Đáp Án Chi Tiết
Số câu hỏi: 24 câuSố mã đề: 1 đềThời gian: 1 giờ
193,983 lượt xem 14,917 lượt làm bài
Xem trước nội dung:
A science class studied the pH strength of acids and bases. The terms strong and weak indicate the ability of acid and base solutions to conduct electricity. Figure 1 displays the pH scale used to rank solutions as acidic or basic, and Table 1 provides an overview of what pH levels are considered strong and weak solutions.
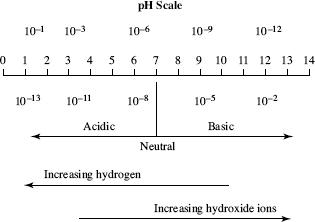
Figure 1
Table 1
| Solution | pH |
| Strong acid | 0–3 |
| Weak acid | 4–6 |
| Neutral | 7 |
| Weak base | 8–10 |
| Strong base | 11–14 |
Experiment 1
Students conducted an experiment to determine the conductivity of acid and base solutions, using a light bulb apparatus (see Figure 2). The light bulb circuit was incomplete. If the circuit is completed by a solution containing a large number of ions, the light bulb will glow brightly, indicating a strong ability to conduct electricity. If the circuit is completed by a solution containing a large number of molecules and either no ions or few ions, the solution does not conduct electricity or conducts it very weakly. Students tested the conductivity of several solutions; the results are reported in Table 2.
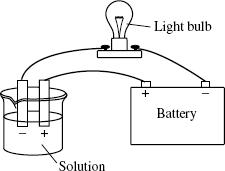
Figure 2
Table 2
| Solution | Acid or Base | Light Bulb |
| H2O | Neutral | No light |
| HCl | Acid | Bright |
| HC2H3O2 | Acid | Dim |
| H2SO4 | Acid | Bright |
| H2CO3 | Acid | Dim |
| NaOH | Base | Bright |
| KOH | Base | Bright |
| NH4OH | Base | Dim |
Experiment 2
Students mixed 50 mL of each chemical with 1 liter of water and then tested the pH of the solution. Table 3 displays the pH found for each chemical.
Table 3
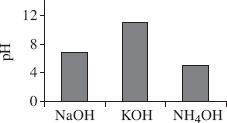
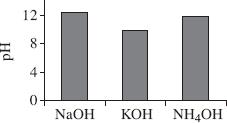
| Chemical | pH |
| HCL | 0.86 |
| HC2H3O2 | 2.92 |
| H2SO4 | 1.29 |
| H2CO3 | 3.73 |
| NaOH | 13.1 |
| KOH | 11.2 |
| NH4OH | 9.2 |
Based on the information presented in Figure 1 and Table 1, a solution with which pH would be the strongest base?
12.5
9.7
7
0.2
According to the results of Experiments 1 and 2, which of the following is true?
The weaker the acid or base, the brighter the light bulb glowed.
The lower the pH of the solution, the brighter the light bulb glowed.
The stronger the acid or base, the brighter the light bulb glowed.
The higher the pH of the solution, the brighter the light bulb glowed.
Based on the results of Experiment 2, KOH would be classified as:
a strong acid.
neutral.
a strong base.
a weak base.
According to the information presented in Figure 1 and Table 3, it can be concluded that which of the following acids contains the most hydrogen?
H23
H24
HC22
HCL
Suppose the students decided to test the conductivity of an additional solution according to the procedures outlined in Experiment 1. They tested a solution of NaOCl, and they found that the solution caused the light bulb to glow brightly. The students would be correct in classifying the NaOCl solution as which of the following?
A strong base
A weak base
A weak acid
Cannot be determined from the given information
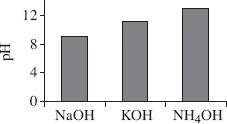
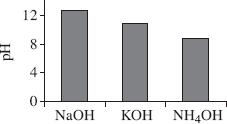
Based on Table 3, which of the following figures best represents the pH values for the 3 bases tested?
Solid-phase microextraction (SPME) is a new technique for extracting volatile organic residue from sediment and water. The technique has several advantages (fast, simple, precise, sensitive), and requires no solvent. For this technique, a thin fused silica fiber coated with a stationary phase is exposed to a sample, either by immersion in a water or air sample or to headspace above an aqueous or solid sample (see Figure 1).
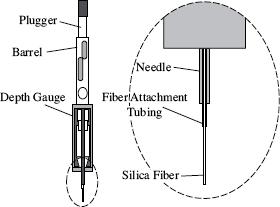
Figure 1: SPME device
Scientists conducted a series of experiments to determine the effectiveness of using SPME to extract white phosphorus (P4) residues from water. All of the experiments were done using headspace immersion.
Experiment 1
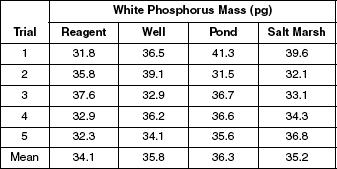
To see how consistent SPME was, scientists conducted five trials using four different types of water (reagent grade, well, pond, salt marsh) at an aqueous concentration of 0.14 μg/L. Table 1 displays the results.
Table 1
Experiment 2
Scientists varied the aqueous concentration and tested four different types of water using SPME, to see the effect on the mass of P4 that could be extracted (see Figure 2).
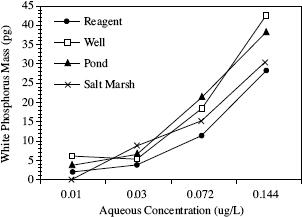
Figure 2
Experiment 3
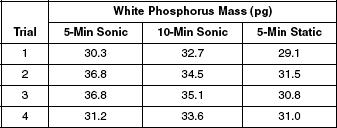
To see if sonication had any effect on the results of SPME, scientists ran four trials, using four identical aqueous samples, at a concentration of 0.14 μg/L. The samples were analyzed using SPME by three methods: five minutes of sonication, ten minutes of sonication, and five minutes static. Table 2 displays the results.
Table 2
According to Figure 2, as the aqueous concentration increased in the test done using salt marsh water, the white phosphorus mass:
decreased, and then increased.
increased and then decreased.
increased.
decreased.
Based on the results from Experiment 3, which trial resulted in the smallest amount of white phosphorus being extracted?
Trial 2 after 5-minutes sonication
Trial 3 after 5-minutes static
Trial 1 after 5-minutes static
Trial 4 after 10-minutes sonication
In order for the scientists to conduct these experiments, it was crucial for them to expose which part of the SPME device to the headspace above the water samples?
The plugger
The needle
The barrel
The silica fiber
A scientist theorized that more white phosphorus would be extracted from a sample if it were exposed to a longer period of sonication. Do the results from Experiment 3 support this theory?
Yes, because in every trial more white phosphorus was extracted after 10-minutes sonication than after 5-minutes sonication.
Yes, because in every trial more white phosphorus was extracted after 5-minutes sonication than after 5-minutes static.
No, because in Trials 2 and 3 more white phosphorus was extracted after 5-minutes sonication than after 10-minutes sonication.
No, because in all trials more white phosphorus was extracted after 5-minutes static than after 5-minutes sonication.
According to Table 1 the mean results demonstrate that the greatest amount of white phosphorus can be extracted from which type of water?
Reagent
Well
Pond
Salt marsh
Based on the results of Experiment 2, if SPME extraction was used on pond water with an aqueous concentration of 0.2 μg/L, it can be inferred that the amount of white phosphorus extracted would be closest to:
45 pg.
100 pg.
30 pg.
5 pg.
The melting point of a substance is the temperature at which the solid phase transitions to the liquid phase. When a substance melts, the attractive forces holding the molecules together are reduced sufficiently to allow the molecules to flow. The stronger the intermolecular forces in solid form, the higher the melting point will be.
The boiling point of a substance is the temperature at which the vapor pressure in the liquid phase equals the atmospheric temperature of its surroundings. When a substance boils, the substance transitions from a liquid phase to a gaseous phase and the intermolecular forces are completely severed. The stronger the attractive forces between the molecules in the liquid phase, the higher the boiling point will be.
Table 1 and Figure 1 show details of some of the elements from the third row of the periodic table.
Table 1
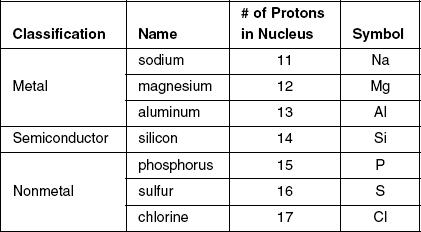
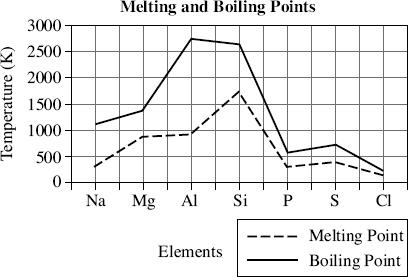
Figure 1
The melting point of silicon is closest to which temperature?
700 K
1200 K
1700 K
2200 K
Based on Table 1 and Figure 1, what can be concluded about the relationship between the number of protons in the nucleus and the attractive forces between molecules?
As the number of protons increase, the intermolecular forces increase.
As the number of protons increase, the intermolecular forces decrease.
As the number of protons increase, the intermolecular forces are unchanged.
There is no relationship between the number of protons and the intermolecular forces.
Based on Figure 1, what is the relationship between melting point (MP) and boiling point (BP)?
There is a direct relationship between melting point and boiling point and the constant of variation is roughly 2. The equation would be MPBP
There is a direct relationship between melting point and boiling point and the constant of variation is roughly 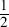 MP
MP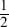 BP
BP
There is an inverse relationship between melting point and boiling point and the constant of variation is roughly 1.5 · 106BP6
There is a direct relationship between melting point and boiling point but no constant of variation exists. When melting point increases, boiling point also increases but every element has a different numerical relationship.
Which element transitions from a liquid phase to a gaseous phase at 500 K?
P
Al
Mg
Na
Do Figure 1 and Table 1 support the conclusion that nonmetals have melting points and boiling points that are closer in temperature than the metals?
Yes. Phosphorus, sulfur, and chlorine all have melting points and boiling points that are less than 500 K apart. Sodium, magnesium, and aluminum have boiling points that are 500 K or more apart.
Yes. Phosphorus, sulfur, and chlorine all have melting points and boiling points that are less than 1000 K apart. Sodium, magnesium, and aluminum have boiling points that are more than 1000 K apart.
No. Silicon’s melting point is 1000 K less than its boiling point. Magnesium’s melting point is 500 K less than its boiling point.
No. There is no general relationship between melting points and boiling points and an element’s classification as a metal or a nonmetal.
From 1971 to 2006, there was a dramatic reduction in the number of feral honeybees in the United States, and a significant, though somewhat gradual, decline in the number of colonies maintained by beekeepers. In early 2007 the rate of attrition reached new proportions, and the term Colony Collapse Disorder (CCD) was coined to describe this sudden decline. CCD is said to have occurred when a bee colony abruptly disappears, with little or no build-up of dead bees in or around the colonies. The cause or causes of the syndrome are not yet well understood. Proposed causes include environmental change-related stress, malnutrition, disease, and pests.
Study 1
In an attempt to quantify the degree and extent of losses experienced in the United States, scientists tracked beekeeping operations with various numbers of colonies between September 2006 and March 2007. For the sake of this study, a colony has encountered CCD when it loses at least 90% of its population in a period of 30 days. This loss must be accompanied by little or no trace of dead bees in or around the hive. Figure 1 displays the total losses experienced by all beekeeping operations.
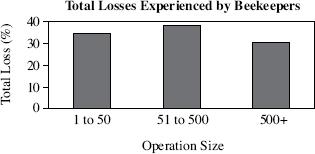
Figure 1
Study 2
Scientists studying CCD surveyed beekeepers whose hives have been affected by the disorder. The beekeepers were asked what they believe caused the CCD in their colonies. Table 1 displays the five most commonly suspected causes of CCD losses and the average percentage of loss experienced by operations of varying sizes due to each suspected cause. The scientists then surveyed beekeepers whose hives had collapsed for reasons unrelated to CCD, asking them the same question. Figure 2 compares the results from the scientists’ two surveys, displaying the total loss experienced due to suspected causes of CCD and non-CCD losses, averaged for operations of all sizes.
Table 1
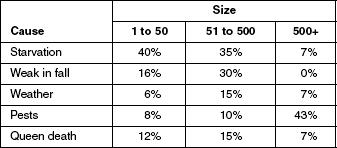
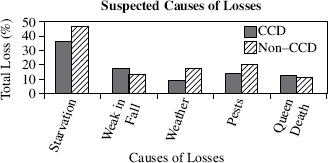
Figure 2
Beekeepers from operations of which size experienced the lowest total losses by percentage?
1 to 50
51 to 500
500+
It cannot be determined from the data provided.
Based on the data in Figure 2, it can be determined that the least common suspected cause of CCD losses was:
starvation.
weak in fall.
weather.
queen death.
According to Study 2, for operations with between 1 and 50 colonies, what was the most common suspected cause of CCD losses?
Starvation
Weak in fall
Weather
Queen death
Prior to conducting the research, four scientists each proposed one of the following hypotheses. Which hypothesis is best supported by the results?
Environmental changes, including shifts in weather patterns, are the predominant causes of CCD-related loss.
An increase in pesticide-resistant pests is the main contributing factor to CCD loss at small beekeeping operations.
The larger a beekeeping operation, the higher the overall CCD loss will be.
A shortage of food sources, especially at smaller beekeeping operations, is leading to an increase in CCD loss.
Figure 2 indicates that, compared with the total loss for non-CCD, the total loss for CCD was higher for which of the following causes?
Starvation, weather, and pests
Weak in fall and queen death
Weak in fall and weather
Starvation and pests
A scientist theorized that the number of CCD losses would be higher than non-CCD losses for beekeeping operations of all sizes. Does the data support this theory?
Yes. For all causes the total loss was higher for CCD than for non-CC
Yes. Starvation was the most common cause of loss regardless of operation size.
No. For many of the causes there were more non-CCD losses than CCD losses.
No. Operations with 51 to 500 colonies experienced the greatest total loss.
Both Study 1 and Study 2 scientists gathered data directly from which of the following sources?
Feral honeybee colonies only
Feral honeybee colonies and non-CCD honeybee colonies
Non-CCD honeybee colonies only
CCD honeybee colonies and non-CCD honeybee colonies
