
ACT Science Practice Test 5
Thời gian làm bài: 1 giờ
Đề thi nằm trong bộ sưu tập: Tuyển Tập Bộ Đề Thi Đại Học Hoa Kỳ (ACT) - Có Đáp Án Chi Tiết
Hãy bắt đầu chinh phục nào!
Xem trước nội dung:
Vasoconstriction involves a narrowing of blood vessels that could lead to poor blood flow in the body if it persists over a long time. Ergotamine is a substance that can cause vasoconstriction. When ergotamine is injected into a normal blood vessel, vasoconstriction occurs quickly at the site of the injection (see Figure 1).
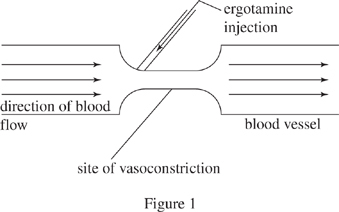
The diameter of the blood vessel at the site of vasoconstriction is less than the diameter of the normal blood vessel, so blood flow has a higher velocity through this narrow site. As a result, the blood pressure in the site of vasoconstriction is less than the blood pressure in the normal blood vessel. Moreover, the higher the velocity of the blood flow through the site of vasoconstriction, the lower the blood pressure at that site.
The percent change in blood pressure (%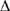 BP) can be defined as:
BP) can be defined as:
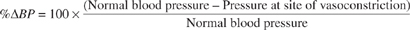
Blood vessel sections of similar diameters were isolated from laboratory rats and %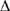 BP was measured over three experiments. When the researchers needed to create a site of vasoconstriction for some of the experimental trials, they would inject ergotamine to induce vasoconstriction within the blood vessel section.
BP was measured over three experiments. When the researchers needed to create a site of vasoconstriction for some of the experimental trials, they would inject ergotamine to induce vasoconstriction within the blood vessel section.
Experiment 1
An artificial heart, which mimics a human's heartbeat, is used to move a constant volume of 500 mL of blood with each beat through four blood vessel sections. These four blood vessel sections were injected with the same amount of ergotamine, leading to sites of vasoconstriction of the same diameter. The rate at which the blood is pumped was varied for the four different blood vessel sections, and the %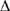 BP values that resulted were measured.
BP values that resulted were measured.
| Table 1 | |
| Rate of artificial heart beat (beats per minute) | %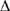 BP BP |
| 60 | 1.2 |
| 90 | 9.3 |
| 120 | 22.3 |
| 150 | 45.1 |
Experiment 2
The artificial heart used in Experiment 1 was then used to pump a constant volume of 500 mL of blood with each beat at a constant rate of 90 beats per minute through five other blood vessel sections. These blood vessel sections were injected with different amounts of ergotamine, resulting in sites of vasoconstriction with different diameters. The %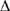 BP values were then measured.
BP values were then measured.
| Table 2 | |
| Diameter of site of vasoconstriction (cm) | %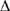 BP BP |
| 0.4 | 40.3 |
| 0.6 | 18.6 |
| 0.8 | 9.3 |
| 1.0 | 4.6 |
| 1.2 | 2.5 |
Experiment 3
The artificial heart used in Experiment 1 was used to pump different volumes of blood at a constant rate of 90 beats per minute through five blood vessel sections with the same diameter at the site of vasoconstriction. The %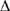 BP values were then measured.
BP values were then measured.
| Table 3 | |
| Volume of blood pumped (mL) | %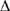 BP BP |
| 400 | 8.4 |
| 450 | 8.8 |
| 500 | 9.3 |
| 550 | 9.7 |
| 600 | 10.2 |
Under the conditions described for Experiment 3, a %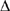 BP of 9.0 would most likely be obtained if the entering volume of blood equaled:
BP of 9.0 would most likely be obtained if the entering volume of blood equaled:
350 m
475 m
550 m
650 m
Based on the results of Experiment 1, if the rate of the artificial heart beat had been less than 60 beats per minute, then the %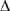 BP would most likely have been:
BP would most likely have been:
less than 1.2.
between 1.2 and 9.3.
between 9.3 and 22.3.
greater than 22.3.
Which of the following is the most likely explanation for the results of Experiment 1? As the rate of the artificial heart beat increases, % BP:
BP:
increases, because the velocity of blood through the site of vasoconstriction increases.
increases, because the velocity of blood through the site of vasoconstriction decreases.
decreases, because the velocity of blood flow through the site of vasoconstriction increases.
decreases, because the velocity of blood flow through the site of vasoconstriction decreases.
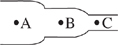
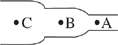
Consider blood flow through three regions of the same blood vessel, each of which has a different diameter. The velocity of blood flow is measured in milliliters per minute (mL/min) and the blood pressure is measured in millimeters of mercury (mmHg), and their values for each of the blood vessel regions are shown in the following table:
| Location | Velocity of blood flow (mL/min) | Blood pressure (mmHg) |
| A | 500 | 31 |
| B | 1,000 | 29 |
| C | 900 | 30 |
Based on the information in the passage about blood flow, which of the following diagrams best represents the relative diameters of the three blood vessel regions?


Based on the results of Experiments 1 and 2, what was the diameter of the site of vasoconstriction in the blood vessel section used in Experiment 3?
0.4 cm
0.6 cm
0.8 cm
1.0 cm
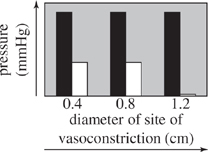
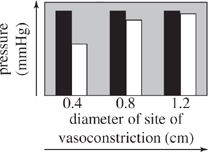
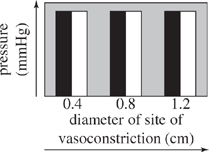
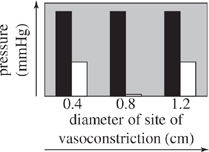
For the blood vessel sections used in Experiment 2 that had sites of vasoconstriction with diameters of 0.4, 0.8, and 1.2 cm, which of the following graphs best displays the comparison between blood pressure at each site of vasoconstriction and blood pressure in the normal region of the blood vessel leading to the site of vasoconstriction?

As the pressure on a gas is increased, the volume of that gas is expected to decrease by an inversely proportional amount. For example, if pressure is doubled the volume is halved. Under certain conditions, the volume of the gas will change by an amount that deviates from an inverse proportion. Various 10.00 L samples of gas were subjected to increases in pressure. Table 1 shows the resulting volume changes at 300°C, while Tables 2 and 3 show the volume changes at 25°C and -200°C, respectively. All pressures are measured in atmospheres (atm).
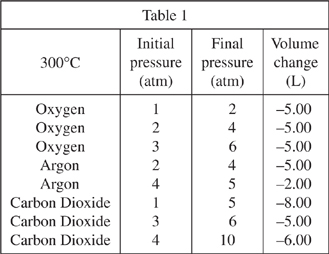
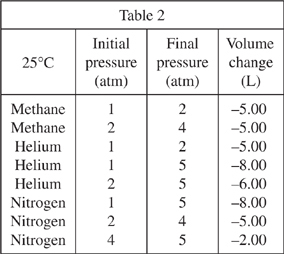
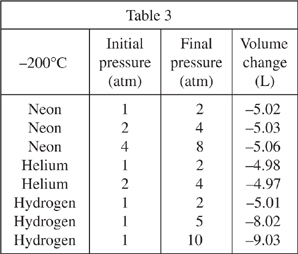
Which of the following gases shown in Tables 1-3 was compressed by the same amount each time the pressure was changed, regardless of its initial pressure?
Helium
Carbon Dioxide
Neon
Oxygen
Which of the following is the best explanation for the change in volume seen in any one of the samples of carbon dioxide in Table 1? As pressure on one sample of carbon dioxide was increased, the volume of that sample:
increased as the molecules of carbon dioxide were forced closer together.
increased as the molecules of carbon dioxide were forced farther apart.
decreased as the molecules of carbon dioxide were forced closer together.
decreased as the molecules of carbon dioxide were forced farther apart.
Based on Table 2, if the sample of nitrogen at a pressure of 4 atm were returned to its initial pressure of 2 atm, the volume would most likely:
decrease by 5.00
decrease by 8.00
increase by 5.00
increase by 8.00
Based on Table 3, if the pressure on a 10.00 L sample of neon gas is increased from 8 atm to 16 atm at a temperature of -200°C, the change in volume will most likely be closest to which of the following?
-5.12 L
-5.06 L
-5.03 L
-5.02 L
A scientist concludes that whenever the pressure on helium is increased, its volume will decrease. Based on Tables 2 and 3, is this a valid conclusion?
Yes; in every trial that the pressure of helium was increased, the change in volume was negative.
No; in every trial that the pressure of helium was increased, the change in volume was positive.
Yes; when the pressure on helium was increased from 1 to 2 atm, its change in volume was positive at 25°C and negative at -200°
No; when the pressure on helium was increased from 1 to 2 atm, its change in volume was negative at 25°C and positive at -200°
There are four planets in our solar system called gas giants: Jupiter, Saturn, Uranus, and Neptune. They are so named because they are composed largely of gases rather than solids. Figure 1 shows how temperatures of the atmospheres of Jupiter, Neptune, and Saturn vary with altitude above the cloud tops. Table 1 gives the composition of the planets in both relative abundance of gases and the altitude at which those gases are most abundant. Table 2 gives what the temperature at the cloud tops would be without greenhouse warming.
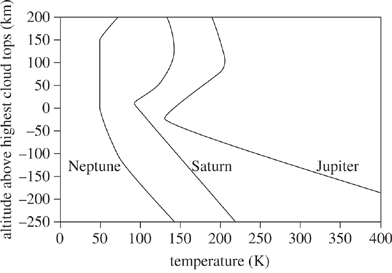
Figure 1
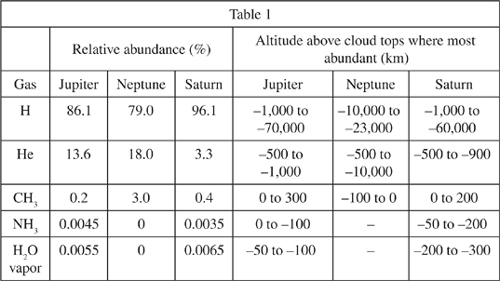
| Table 2 | |
| Planet | Temperature at cloud tops without greenhouse warming (K) |
| Jupiter Neptune Saturn | 100 50 25 |
According to Figure 1, the temperature of Neptune remains the same as altitude above the highest cloud tops increases from:
-250 km to -200 km.
-150 km to -50 km.
0 km to 100 km.
150 km to 200 km.
According to Figure 1, the temperature of Jupiter changes the most between:
-150 km and -50 km.
-50 km and 50 km.
50 km and 100 km.
100 km and 200 km.
Considering only the gases listed in Table 1, which gas is more abundant in the atmosphere of Jupiter than in the atmosphere of either Neptune or Saturn?
H
CH3
NH3
He
Based on Table 2, the average temperature at Saturn's cloud tops without greenhouse warming is how many degrees cooler than the temperature given in Figure 1?
5 K
25 K
75 K
150 K
Which of the following statements about H and He in the atmospheres of the 3 planets is supported by the data in Table 1?
Both Saturn and Neptune have a higher relative abundance of He than of
Both Saturn and Jupiter have a higher relative abundance of He than of
Both Jupiter and Neptune have an equivalent relative abundance of He and
Both Saturn and Neptune have a lower relative abundance of He than of
Nuclear fission occurs when the nucleus (central core) of an atom splits into multiple parts. This splitting is accompanied by the release of a large amount of energy, as in nuclear weapons and nuclear power plants.
A chemical element is said to be radioactive if it is prone to fission. Fission is often the result of the nucleus of a radioactive atom absorbing a free neutron (an uncharged nuclear particle). When a fission event occurs, the nucleus often splits into two new nuclei and produces free neutrons. This process generates the possibility of a chain reaction. If, on average, a fission event produces one neutron and that neutron causes another nucleus to fission, the reaction is said to be critical; that is, it will sustain itself, but not increase in magnitude. If one fission event releases more free neutrons than are required to initiate another fission event, the reaction is said to be supercritical; that is, it will sustain and increase in magnitude. If more neutrons are required to initiate a fission event than are released in fission, the reaction is said to be subcritical: the reaction will not sustain itself.
Many factors affect how many neutrons from each fission event will trigger another fission event. The most important factor is the mass (m) of the substance. The criticality of a substance also depends on the substance's purity, shape, density, temperature, and whether or not it is surrounded by a material that reflects neutrons.
In a nuclear weapon, a radioactive substance is made highly supercritical. One of the primary challenges in building a nuclear weapon is keeping the radioactive material subcritical prior to detonation, then upon detonation, keeping it supercritical for a long enough period of time for all of the material to fission before it is blown apart by the energy of the blast. A fizzle occurs when a nuclear weapon achieves supercriticality but is blown apart before all of the radioactive material fissions.
The first nuclear weapons were made of enriched uranium, or U-235. The density (ρ) of U-235 under normal conditions is 19.1 g/cm3. For U-235 to attain a supercritical state, the product of its mass and density must exceed 106 g2/cm3. If it is assembled over too long a time (t), it will achieve slight supercriticality and then fizzle. Therefore, the speed of assembly (measured as t divided by ρ), must be less than 10-5 sec × cm3/g (Michelson's Criterion).
Two schemes for the assembly of a supercritical amount of U-235 that avoid fizzle are discussed below.
Gun-Type Weapon
At one end of a tube, similar to a gun barrel, is a hollow, subcritical cylinder of U-235 with a mass of 48 kg; on the other end is a subcritical pellet of U-235 with a mass of 12 kg. The pellet is propelled by a small explosion down the tube and into the cylinder of U-235. The combined mass of the two pieces of U-235 is great enough to induce a supercritical state. Since the combined cylinder of U-235 is at or near normal density, the assembly process must be completed in less than 2 × 10-4sec to meet Michelson's Criterion.
Implosion-Type Weapon
A 15-kg sphere of U-235 is surrounded by explosives. When the explosives are simultaneously detonated, the U-235 is compressed in order to achieve supercriticality. The explosives are designed to compress the U-235 to a density of approximately 70 g/cm3 in less than 10-7 sec.
For both types of weapon, avoiding fizzle is difficult because:
the mass of U-235 must be large.
2 separate pieces of U-235 must be brought together.
U-235 is highly unstable.
of the speed with which the U-235 must be assembled.
Comparing the mass of uranium used in the two types of weapons reveals that:
the mass of U-235 used in the implosion-type weapon is less than the mass of U-235 used in the gun type weapon.
the mass of U-235 used in the implosion-type weapon is greater than the mass of U-235 used in the gun type weapon.
the mass of U-235 used in the implosion-type weapon is greater in some cases and less in some cases than the mass used in the gun-type weapon.
the mass of U-235 used in both weapons is approximately the same.
Both types of weapons use explosives in order to:
increase the heat of the U-235.
release the nuclear energy of the weapon from the confinement of the bomb's casing.
achieve supercriticality of U-235.
generate neutrons to start the chain reaction.
For an implosion-type weapon, when U-235 has reached supercriticality, to which of the following is the value of ρ closest?
10-33
0.1 g/cm3
100 g/cm3
1063
In the implosion-type weapon, the explosives are used to:
trigger the first fission events.
heat the U-235 so it will become supercritical.
increase the density of U-235.
produce additional damage.
In order to achieve a supercritical state just before detonation, both methods:
increase the product of the mass and density of the U-235.
decrease the product of the mass and density of the U-235.
increase the amount of U-235 in the weapon.
decrease the time necessary for all the U-235 to fission.
Scientists are trying to build a bomb using only 8 kg of U-235. Presently they can achieve a ρ of 150 g/cm3 with t = 10-2 sec. Which of the following changes would be the most likely to get the weapon to meet Michelson's Criterion?
Decrease both t
Decrease t
Increase t
Increase t
Xem thêm đề thi tương tự

12 câu hỏi 1 mã đề 1 giờ
214,108 lượt xem 115,283 lượt làm bài

13 câu hỏi 1 mã đề 1 giờ
217,163 lượt xem 116,928 lượt làm bài

13 câu hỏi 1 mã đề 1 giờ
206,349 lượt xem 111,104 lượt làm bài

10 câu hỏi 1 mã đề 1 giờ
218,800 lượt xem 117,810 lượt làm bài

10 câu hỏi 1 mã đề 1 giờ
217,602 lượt xem 117,166 lượt làm bài

12 câu hỏi 1 mã đề 1 giờ
208,788 lượt xem 112,420 lượt làm bài

11 câu hỏi 1 mã đề 1 giờ
207,645 lượt xem 111,804 lượt làm bài

12 câu hỏi 1 mã đề 1 giờ
206,527 lượt xem 111,202 lượt làm bài

11 câu hỏi 1 mã đề 1 giờ
209,139 lượt xem 112,609 lượt làm bài

