
ACT Science Practice Test 56
Thời gian làm bài: 1 giờ
Đề thi nằm trong bộ sưu tập: Tuyển Tập Bộ Đề Thi Đại Học Hoa Kỳ (ACT) - Có Đáp Án Chi Tiết
Hãy bắt đầu chinh phục nào!
Xem trước nội dung:
PASSAGE V
Near the end of the 19th century, British engineer Osborne Reynolds ran a set of experiments to observe and predict the transition between laminar (steady) and turbulent flow of a liquid through a pipe. In Reynolds' experiments, dye was forced through a liquid to show visually when the flow changed from laminar to turbulent. Laminar flow is common only in cases in which the flow channel is relatively small, the fluid is moving slowly, and its viscosity (the degree to which a fluid resists flow under an applied force) is relatively high. In turbulent flow, the speed of the fluid at any given point is continuously undergoing changes in both magnitude and direction. Reynolds demonstrated that the transition from laminar to turbulent flow in a pipe depends upon the value of a mathematical quantity equal to the velocity of flow (V) times the diameter of the tube (D) times the mass density (ρ) of the fluid divided by its absolute viscosity (μ). The "Reynolds number," as it is called, is determined by the following equation:
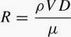
Several students designed similar experiments to observe flow rates of different liquids. To conduct the experiments, the students were given the following apparatus:
o Liquid supply tank with clear test section tube and 'bell mouth' entrance
o 1 Rotameter to measure the velocity of flow (flow rate)
o Tap water
o Motor oil
o 4, 10-ft long smooth pipes of various diameters: 0.25-inch, 0.50-inch, 0.75-inch, 1.0-inch
Figure 1 illustrates an approximation of the set-up of each experiment.
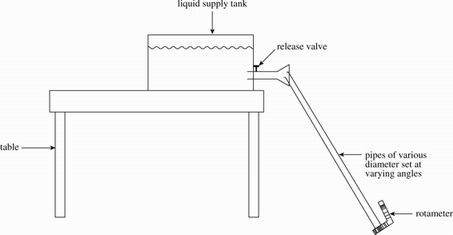
Figure 1
Figure 2 shows approximate viscosities of the water and motor oils used in the experiments.

Figure 2
Experiment 1
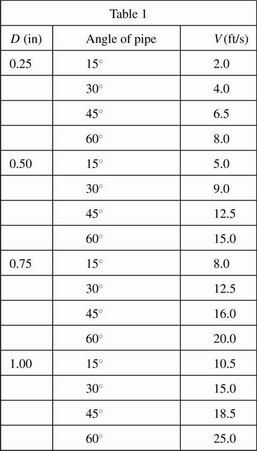
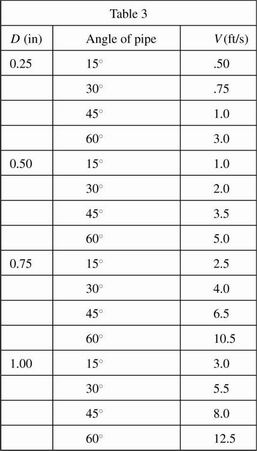
In Experiment 1, students began with a pipe of diameter 0.25 inches. The pipe was set first at a 15° angle and tap water was released steadily from the tank into the pipe. The velocity of flow (V) was measured. The pipe was then set at a 30° angle, a 45° angle, and a 60° angle, water was released steadily from the tank into the pipe, and the velocity of flow was measured. The process was then repeated for each diameter of pipe using the same amount of water each time. All data were recorded in Table 1. Temperature of the water was held constant at 20°C.
Experiment 2
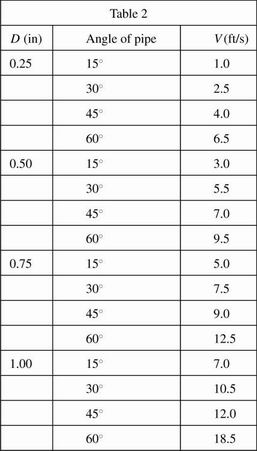
In the second experiment, the tap water was replaced by Motor Oil A and the processes were repeated. The results are given in Table 2.
Experiment 3
In a third experiment, the tap water was replaced by Motor Oil B and the processes were repeated.
Information in the passage and the results of the experiments indicate which of the following? Compared to tap water, Motor Oil A:
has a lower viscosity.
has a higher viscosity.
has an overall higher flow rate.
does not exhibit laminar flow.
Based on Experiment 1, the relationship between the angle of the pipe and the velocity of flow:
is indirect.
is direct.
varies, but with no general trend.
cannot be determined.
According to the passage, laminar flow was most likely to be observed under which of the following conditions?
Experiment 1, pipe diameter of 0.50 in, pipe angle of 45°.
Experiment 3, pipe diameter of 0.50 in, pipe angle of 15°.
Experiment 1, pipe diameter of 1.0 in, pipe angle of 30°.
Experiment 3, pipe diameter of 1.0 in, pipe angle of 60°.
Which of the following conclusions is best supported by information in the passage? As viscosity increases:
laminar flow decreases.
velocity of flow increases.
velocity of flow decreases.
laminar flow cannot be measured.
In Experiment 1, at a 30° angle, flow rate would most likely have been approximately 6.0 ft/s for which new pipe diameter?
0.4 in
0.6 in
0.8 in
1.2 in
All of the experimental factors were identical EXCEPT:
the length of the pipes.
the amount of liquid released into each pipe.
the type of liquid used.
the apparatus used to measure flow rate.
PASSAGE VI
Certain preservatives known as sulfites are often added to fruit products to keep the fruit fresher longer. Use of sulfites is controversial because studies have linked sulfites to severe reactions in some asthmatics. Students performed 2 experiments to measure sulfite levels.
Experiment 1
Four solutions, each containing a different amount of sulfite dissolved in H2O were prepared. A coloring agent was added that binds with sulfite to form a red compound that strongly absorbs light of a specific wavelength, and each solution was diluted to 100 mL. A blank solution was prepared in the same manner, but no sulfite was added. A colorimeter (a device that measures how much light of a selected wavelength is absorbed by a sample) was used to measure the absorbance of each solution. The absorbances were corrected by subtracting the absorbance of the blank solution from each reading (see Table 1 and Figure 1).
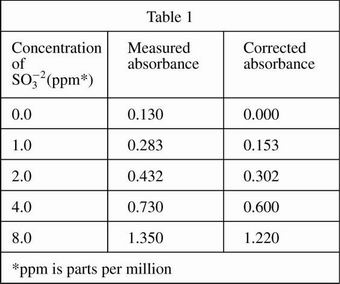
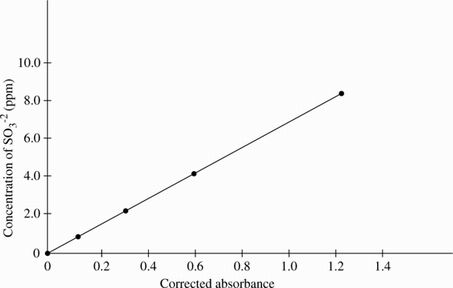
Figure 1
Experiment 2
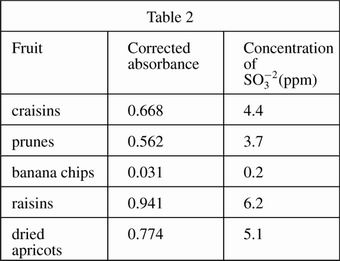
A 100 g fruit sample was ground in a food processor with 50 mL of H2O and the mixture was filtered. The food processor and remaining fruit were then washed with H2O, these washings were filtered, and the liquid was added to the sample solution. The coloring agent was added and the solution was diluted to 100 mL. The procedure was repeated for several fruits, and the absorbances were measured (see Table 2).
Based on the results of Experiment 1, if the concentration of sulfite in a solution is doubled, then the corrected absorbance of the solution will approximately:
remain the same.
halve.
double.
quadruple.
A sample of dried pineapple was also measured in Experiment 2 and its corrected absorbance was determined to be 0.603. Which of the following correctly lists prunes, apricots, and dried pineapple in decreasing order of corrected absorbance?
Prunes, dried apricots, dried pineapple.
Dried pineapple, apricots, prunes.
Prunes, dried pineapple, dried apricots.
Dried apricots, dried pineapple, prunes.
Based on the results of Experiment 1, if a solution with a concentration of 1.5 ppm sulfite had been tested, the corrected absorbance would have been closest to which of the following values?
0.160
0.240
0.300
0.360
If Experiments 1 and 2 were repeated using a different coloring agent that produces a different color when it binds with sulfite, which of the following changes in procedure would be necessary?
The new coloring agent should be added to the blank solution, but not to the sample solutions.
Both of the coloring agents should be added to the blank solution and to all of the samples.
The absorbance of the blank solution made with the new coloring agent should be added to the measured absorbances.
The colorimeter should be set to measure at a different wavelength of light.
Based on the results of Experiments 1 and 2, if the measured absorbances for the fruits tested in Experiment 2 were compared with their corrected absorbances, the measured absorbances would be:
higher for all of the fruits tested.
lower for all of the fruits tested.
lower for some of the fruits tested, higher for others.
the same for all of the fruits tested.
If some of the water-soluble contents found in all of the fruits tested in Experiment 2 absorbed light of the same wavelength as the compound formed with sulfite and the coloring agent, how would the measurements have been affected? Compared to the actual sulfite concentrations, the sulfite concentrations apparently measured would be:
higher.
lower.
the same.
higher for some of the fruits, lower for others.
Xem thêm đề thi tương tự

12 câu hỏi 1 mã đề 1 giờ
214,108 lượt xem 115,283 lượt làm bài

24 câu hỏi 1 mã đề 1 giờ
219,327 lượt xem 118,090 lượt làm bài

11 câu hỏi 1 mã đề 1 giờ
209,258 lượt xem 112,672 lượt làm bài

13 câu hỏi 1 mã đề 1 giờ
217,163 lượt xem 116,928 lượt làm bài

13 câu hỏi 1 mã đề 1 giờ
206,349 lượt xem 111,104 lượt làm bài

23 câu hỏi 1 mã đề 1 giờ
218,481 lượt xem 117,635 lượt làm bài

22 câu hỏi 1 mã đề 1 giờ
219,703 lượt xem 118,293 lượt làm bài

24 câu hỏi 1 mã đề 1 giờ
218,957 lượt xem 117,894 lượt làm bài

24 câu hỏi 1 mã đề 1 giờ
219,404 lượt xem 118,132 lượt làm bài
