
ACT Science Practice Test 52
Đề thi nằm trong bộ sưu tập: Tuyển Tập Bộ Đề Thi Đại Học Hoa Kỳ (ACT) - Có Đáp Án Chi Tiết
Số câu hỏi: 10 câuSố mã đề: 1 đềThời gian: 1 giờ
218,852 lượt xem 16,830 lượt làm bài
Xem trước nội dung:
PASSAGE IV
When connection to a municipal water system is not feasible, wells are drilled to access ground water. Engineers employed by a company interested in developing a remote plot of land conducted studies to compare the water quality of 2 possible well locations on the land. Water quality is determined by a number of factors, including the levels of nitrates, lead, microbes, pH, "hardness" (calcium carbonate), and alkalinity. The water samples were kept at a constant temperature of 72°F throughout the study. The results in Table 1 show the readings of each test for the two different 100 mL samples of water, as well as the ideal level, or concentration, for each chemical.
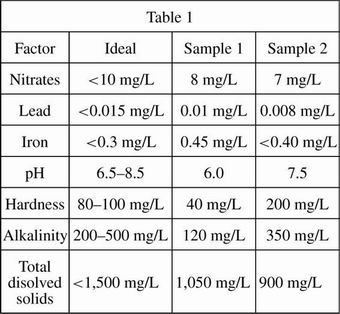
The pH scale measures how acidic or basic a substance is on a scale of 0 to 14. Lower numbers indicate increasing acidity and higher numbers indicate increasing basicity. The normal pH level of groundwater systems is between 6 and 8.5. Water with a low pH (<6.5) could be acidic, soft, and corrosive, and could contain elevated levels of toxic metal that might cause premature damage to metal piping. Water with a pH >8.5 could indicate that the water is hard. Hard water does not pose a health risk, but can cause mineral deposits on fixtures and dishes and can have a bad taste and odor.
Alkalinity is the water's capacity to resist decreases in pH level. This resistance is achieved through a process called buffering (a buffered solution resists changes in pH until the buffer is used up). Alkalinity of natural water is determined by the soil and bedrock through which it passes. The main sources for natural alkalinity are rocks that contain carbonate, bicarbonate, and hydroxide compounds. These compounds, however, also cause hardness, which is less desirable in a drinking source. To illustrate the affect of alkalinity on pH stability, acid was added to two 100 milliliters sample solutions that initially had a pH of 6.5. The solution in Figure 1A had an alkalinity level of 200 mg/L while the solution in Figure 1B tested at zero alkalinity. The pH of the two solutions was recorded after every addition of acid and the results are shown in the figures below.
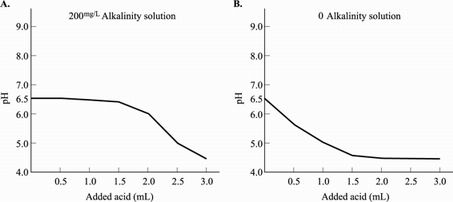
Figure 1
Which of the following statements best describes the concentration of lead in Sample 1?
The concentration of lead in Sample 1 is above the ideal level.
The concentration of lead in Sample 1 may be corrosive to surfaces.
The concentration of lead in Sample 1 is at or below the ideal level.
The concentration of lead in Sample 1 is less than the concentration of lead in Sample 2.
An ideal alkalinity level prevents pH levels from becoming too low. Which statement is best supported by this fact? When testing drinking water:
an alkalinity test is not necessary.
an alkalinity level above 500 is ideal.
ideal samples will have levels similar to that of Sample 1.
a proper alkalinity level can prevent water from becoming overly corrosive.
The test results of Sample 1 indicate that:
the water from Sample 1 is probably balanced and safe.
the water from Sample 1 is too acidic and corrosive.
alkalinity levels are high enough to prevent it from becoming overly acidic.
the water tested in Sample 1 is hard water.
Based on the test results, Sample 2 is acceptable as a water source as long as the developers:
are willing to accept high iron levels and hard water.
are willing to accept high lead levels and soft water.
are willing to accept high alkalinity levels and soft water.
treat the water to reduce its corrosive nature.
Suppose chemicals could be added to treat the high iron levels in either sample. The chemical additive would be safe to use in Sample 2 and not safe to use in Sample 1 if:
the chemical additive caused a drastic increase in pH levels in unbuffered solutions.
the chemical additive caused an increase in water hardness levels.
the chemical additive caused a decrease in total dissolved solids.
the chemical additive increased the amount of dissolved solids by at least 200 mg/
PASSAGE V
Some students performed 3 studies to measure the average speed on a flat surface of a gas-powered golf cart. Each study was conducted on a fair day with no wind. A 500-foot long flat surface was measured, and the cart's travel time was measured from start to finish with a stopwatch. The cart was not modified in any way, the same driver was used each time, and the cart's fuel tank was filled before each trial.
Study 1
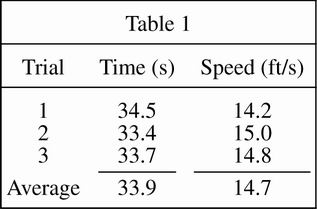
The students first drove the cart on a smooth asphalt road. One student drove the cart as the other student started the stopwatch. The student stopped the stopwatch as the cart crossed the 500-foot mark. The students calculated the results of three separate trials and averaged the results (see Table 1).
Study 2
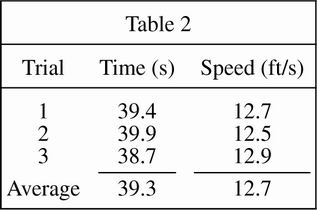
The students repeated the procedure used in Study 1, except the cart was driven on the fairway (very short, well-groomed grass). The results are shown in Table 2.
Study 3
The students repeated the procedure used in Study 1, except they drove the cart through the rough (thick, long grass). The results are shown in Table 3.
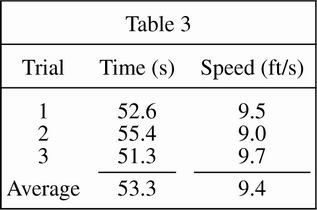
The highest average speeds resulted from using which surface?
Fairway
Rough
Asphalt
The speeds remained constant.
According to Table 1, the average speed for all three trials is:
less than the speed measured in Trial 1.
greater than the speed measured in Trial 3.
greater than the speed measured in Trial 2.
less than the speed measured in Trial 3.
According to Tables 1, 2, and 3:
the average speed of the cart in the rough is approximately two-thirds of the average speed of the cart on asphalt.
the average speed of the cart on the fairway is approximately one-third of the average speed of the cart on asphalt.
the average speed of the cart in the rough is approximately two-thirds of the average speed of the cart on the fairway.
the average speed of the cart on asphalt is approximately two-thirds of the average speed of the cart in the rough.
According to the passage, which of the following was the independent variable in each of the studies?
The surface upon which the golf cart was driven.
The amount of fuel in the tank of the golf cart.
The average speed of the golf cart.
The number of trials conducted.
During which of the following was the average travel time of the car the slowest?
Study 1, Trial 2.
Study 2, Trial 2.
Study 3, Trial 2.
Study 3, Trial 3.
