
ACT Science Practice Test 41
Thời gian làm bài: 1 giờ
Đề thi nằm trong bộ sưu tập: Tuyển Tập Bộ Đề Thi Đại Học Hoa Kỳ (ACT) - Có Đáp Án Chi Tiết
Hãy bắt đầu chinh phục nào!
Xem trước nội dung:
Two experiments were performed to study Newton’s laws of motion using air track gliders. The friction between the gliders and the air track is nearly zero. Each glider has a spring attached on each end.
Experiment 1
Two identical gliders were placed on an air track as shown in Figure 1. The gliders’ springs were then compressed completely together along with the gliders and released. When the gliders were pushed apart by their springs, the data in Table 1 was recorded. The zero position was recorded as the far-left point where Glider 1 is shown in Figure 1.
Table 1
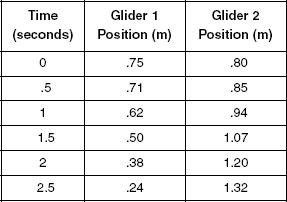
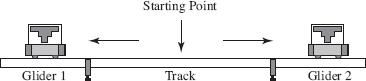
Figure 1
Experiment 2
The same experiment was performed but Glider 3 was a double-weight glider in the first trial. In the second trial, Glider 4, a triple-weight glider, was tested.
Table 2
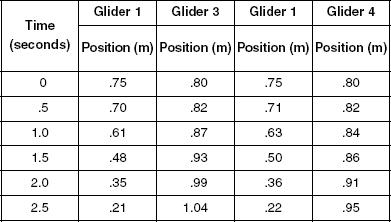
The velocity for each trial was calculated and the ratio of Glider 1 to the other glider was graphed and shown in Figure 2.
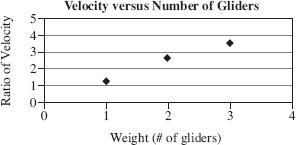
Figure 2
Suppose another trial was run with Glider 1 and a Glider 4 that weighs 4 times Glider 1. The ratio of the velocities would be closest to what number?
1
2
3
4
In Experiment 1, how many times a second did the experimenters record the position of the gliders?
Once per second
Twice per second
Three times per second
Four times per second
Based on Tables 1 and 2, how did the total distance traveled by both gliders vary against the weight of the gliders?
Total Distance----Total Weight
Decreased Increased
Increased Decreased
Increased Increased
Unchanged Increased
Based on Table 2, how far had Glider 4 traveled after 1.5 seconds?
.02 m
.06 m
.80 m
.86 m
What distance was separating the gliders before they were released in each experiment?
0 m
.02 m
.05 m
.08 m
Since carbon is essential to life on Earth, understanding the global carbon cycle can provide valuable information. Through the process of photosynthesis, plants on land or algae living in water convert carbon dioxide into organic products. Through the process of respiration, organisms convert these organic compounds into energy and release carbon dioxide. Figure 1 displays the global carbon cycle.
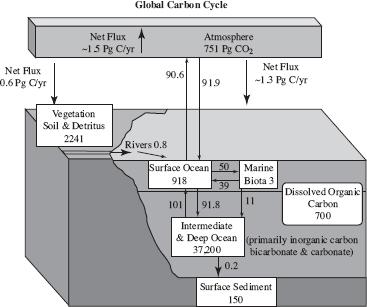
Figure 1
A significant amount of carbon is released through the burning of fossil fuels. Some of this carbon is stored in the atmosphere, while a significant portion is stored in ocean waters and sediments. Carbon in the ocean is described as dissolved inorganic carbon, particulate inorganic carbon, particulate organic carbon (POC), and dissolved organic carbon (DOC). Particulates are defined as those larger than 0.2 micrometers, whereas dissolved matter is that smaller than 0.2 micrometers (see Figure 2).
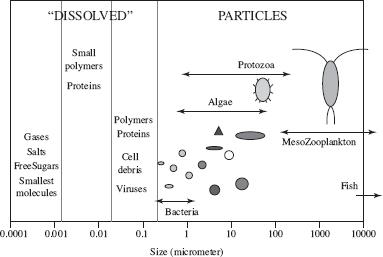
Figure 2
Chromophoric dissolved organic matter (CDOM) is the colored portion of DOC. It is colored because it intensely absorbs violet and blue light. Figure 3 displays DOC and CDOM absorption in the tropical North Atlantic Ocean.
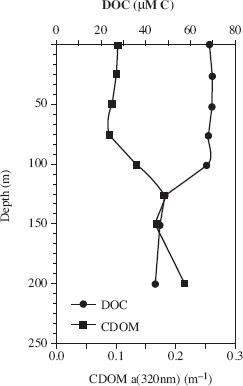
Figure 3
Based on Figure 1, how much carbon is transmitted from the ocean to the atmosphere?
91.9 Pg C/yr
90.6 Pg C/yr
918 Pg C/yr
700 Pg C/yr
According to Figure 2, which of the following is NOT considered a dissolved carbon?
Proteins
Salts
Algae
Viruses
Based on Figure 3, which of the following statements best describes the relationship between ocean depth and amount of DOC?
As the ocean depth increased, the amount of DOC remained fairly constant, then decreased steadily, then began to increase again.
As the ocean depth increased, the amount of DOC steadily decreased.
As the ocean depth increased, the amount of DOC remained fairly constant, then decreased steadily, then became fairly constant again.
As the ocean depth increased, the amount of DOC steadily increased.
Suppose a fragment of organic carbon was found in the ocean and measured approximately 0.01 micrometers. It would mostly likely be featured in Figure 2 by which of the following?
Gases
Protozoa
Bacteria
Small polymers
At an ocean depth of 100 m, there is approximately how much CDOM, according to Figure 3?
0.25
0.12
40
70
Which of the following fragments, if found in the ocean, would be described as dissolved inorganic carbon?
Bacteria
Protozoa
Viruses
Polymers
Three substances have been shown to inhibit respiration in various organisms: hydrocyanic acid, hydrogen sulfide, and carbon monoxide. An experiment was conducted to determine the effect that each of these substances has on the respiration of the green alga Chlorella. Figure 1 shows the results.
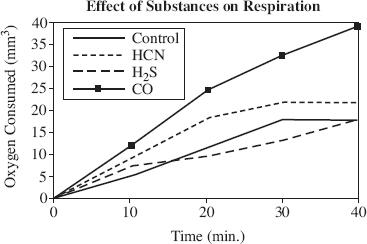
Figure 1
Researcher 1 argued that glucose would have a contradictory effect on the alga, so that if the Chlorella were suspended in a solution containing 1 percent glucose, there would be less of an effect on respiration. Figure 2 displays the results of the experiment.
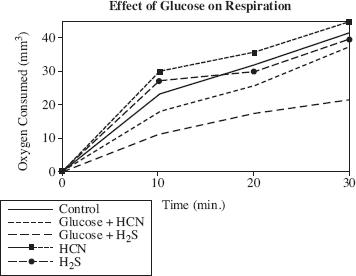
Figure 2
Researcher 2 hypothesized that light may play an active part in respiration of Chlorella, so an experiment was done to measure the effect of successive periods of light and darkness on the respiration of cells suspended in carbon monoxide and in nitrogen. The results are shown in Figure 3.
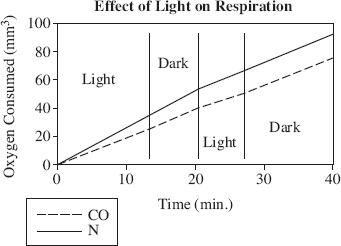
Figure 3
The theories of the two researchers are similar in that both researchers believe that:
glucose will increase the respiration of Chlorella
HCN and H2Chlorella
an additional factor will have an effect on the respiration of Chlorella
CO and N also affect the respiration of Chlorella
According to Figure 1, Chlorella would typically consume about how much oxygen after half an hour?
10 mm3
18 mm3
22 mm3
35 mm3
If Researcher 1 was correct about the effect of glucose on the respiration of Chlorella, then based on the information in Figure 2, Researcher 1 would most likely predict that Chlorella suspended in a solution containing 2 percent glucose and HCN would consume how much oxygen after 20 minutes?
15 mm3
25 mm3
30 mm3
40 mm3
Does the data in Figure 3 support Researcher 2’s hypothesis?
Yes. It shows that light is necessary for the respiration of Chlorella
Yes. It shows that equal respiration occurs in the darkness and in light.
No. More respiration of Chlorella
No. Varying the amount of light did not yield significantly different rates of respiration.
Suppose a third researcher studied the effect of glucose and CO on the respiration of Chlorella, using the same conditions and methods as Researcher 1. After 20 minutes the results showed that the sample suspended in CO had consumed 50 mm3 of oxygen, while the sample suspended in a mixture of CO and glucose had consumed 60 mm3 of oxygen. How would this experiment most likely affect the researchers’ viewpoints?
It would weaken Researcher 2’s viewpoint only.
It would weaken Researcher 1’s viewpoint only.
It would strengthen both researchers’ viewpoints.
It would have no effect on either researcher’s viewpoint.
According to Figure 2, which substance had the LEAST effect on respiration of Chlorella after 30 minutes?
HCN
H2
Glucose + HCN
Glucose + H2
According to Researcher 2, experiment findings indicate the following is FALSE regarding the relationship between Chlorella cells suspended in carbon monoxide and Chlorella cells suspended in nitrogen EXCEPT:
A catalyst is a substance that speeds up a reaction, but is chemically unchanged at the end of the reaction. Catalysts can be divided into two main types—heterogeneous and homogeneous. In a heterogeneous reaction, the catalyst is in a different phase (such as solid, liquid, or gas) from the reactants. In a homogeneous reaction, the catalyst is in the same phase as the reactants. Most examples of heterogeneous catalysis go through the same stages (see Table 1).
Table 1
Stage | Description |
1 | One or more of the reactants are absorbed onto the surface of the catalyst. |
2 | There is some sort of interaction between the surface of the catalyst and the reactant molecules, which makes them more reactive. |
3 | The reaction happens. |
4 | The product molecules are desorbed, meaning that the product molecules break away. |
Students did experiments to convert propanol to propene using alumina beads, and then to convert propene to propanol using a palladium catalyst.
Experiment 1
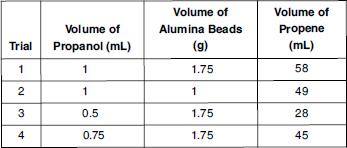
Two glass syringes were connected to an aluminabead catalyst tube (see Figure 1). The 1 mL syringe was filled with 1 mL of propanol. Next, the apparatus was held above a burner’s flame and the alumina-bead catalyst tube was gently heated while the liquid propanol was slowly introduced into the catalyst tube. The liquid flowed through the tube until it hit the hot region. Then it vaporized, reacted with the catalyst, and exited the catalyst tube as gaseous propene into the 60-mL receiver syringe. The procedure was repeated with various amounts of propanol and alumina beads and the amount of gaseous propene collected was recorded (see Table 2).
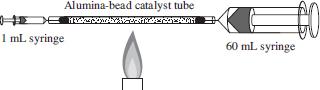
Figure 1
Table 2
Experiment 2
A reactant syringe was filled with equal volumes of hydrogen and propene. The reactant syringe and receiver syringe were connected to the catalyst tube filled with solid palladium as shown in Figure 2. Then the hydrogenpropene mixture was slowly passed over the catalyst, the reaction occurred, and the propane was collected in the receiver syringe. The procedure was repeated several times, varying the amount of time the reactant was passed over the catalyst. The results are shown in Table 3.

Figure 2
Table 3
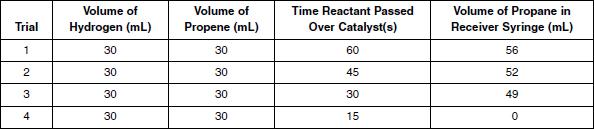
In Experiment 2, as the amount of time the reactant was passed over the catalyst decreased, the volume of propane created:
increased only.
decreased only.
increased and then remained constant.
decreased and then remained constant.
Which is the most likely explanation for why 0 mL of propane was produced in Trial 4 of Experiment 2?
There was not enough volume of hydrogen and propene to create a reaction.
Fifteen seconds was too long a time for the reactant to pass over the catalyst.
There was not enough volume of palladium in the catalyst tube to create a reaction and produce propane.
The reactant was not allowed enough time to interact with the catalyst; therefore no reaction occurred and no propane was produced.
What type of catalyst was used in Experiment 1?
It cannot be determined from the information given.
A liquid catalyst
A heterogeneous catalyst
A homogeneous catalyst
In Experiment 2, the hydrogen-propene mixture turns into propane at what stage of the catalysis?
Stage 1
Stage 2
Stage 3
Stage 4
Based on the data in Table 2, which two trials illustrate the effect that varying the volume of the catalyst has on the volume of propene produced?
Trials 1 and 2
Trials 1 and 3
Trials 3 and 4
Trials 2 and 4
Which of the following best describes what occurred to the plunger of the 60-mL syringe during Experiment 1? When propanol was injected into the catalyst tube, the distance between the end of the plunger and the syringe tip:
decreased slowly over 30 seconds.
increased immediately, and then decreased as the reaction occurred.
remained in place until the reaction occurred, and then decreased.
remained in place until the reaction occurred, and then increased.
Xem thêm đề thi tương tự

12 câu hỏi 1 mã đề 1 giờ
214,108 lượt xem 115,283 lượt làm bài

24 câu hỏi 1 mã đề 1 giờ
219,326 lượt xem 118,090 lượt làm bài

11 câu hỏi 1 mã đề 1 giờ
209,258 lượt xem 112,672 lượt làm bài

13 câu hỏi 1 mã đề 1 giờ
217,162 lượt xem 116,928 lượt làm bài

13 câu hỏi 1 mã đề 1 giờ
206,348 lượt xem 111,104 lượt làm bài

23 câu hỏi 1 mã đề 1 giờ
218,480 lượt xem 117,635 lượt làm bài

22 câu hỏi 1 mã đề 1 giờ
219,702 lượt xem 118,293 lượt làm bài

24 câu hỏi 1 mã đề 1 giờ
219,404 lượt xem 118,132 lượt làm bài

26 câu hỏi 1 mã đề 1 giờ
219,188 lượt xem 118,020 lượt làm bài

